2025-09-12 清華大学
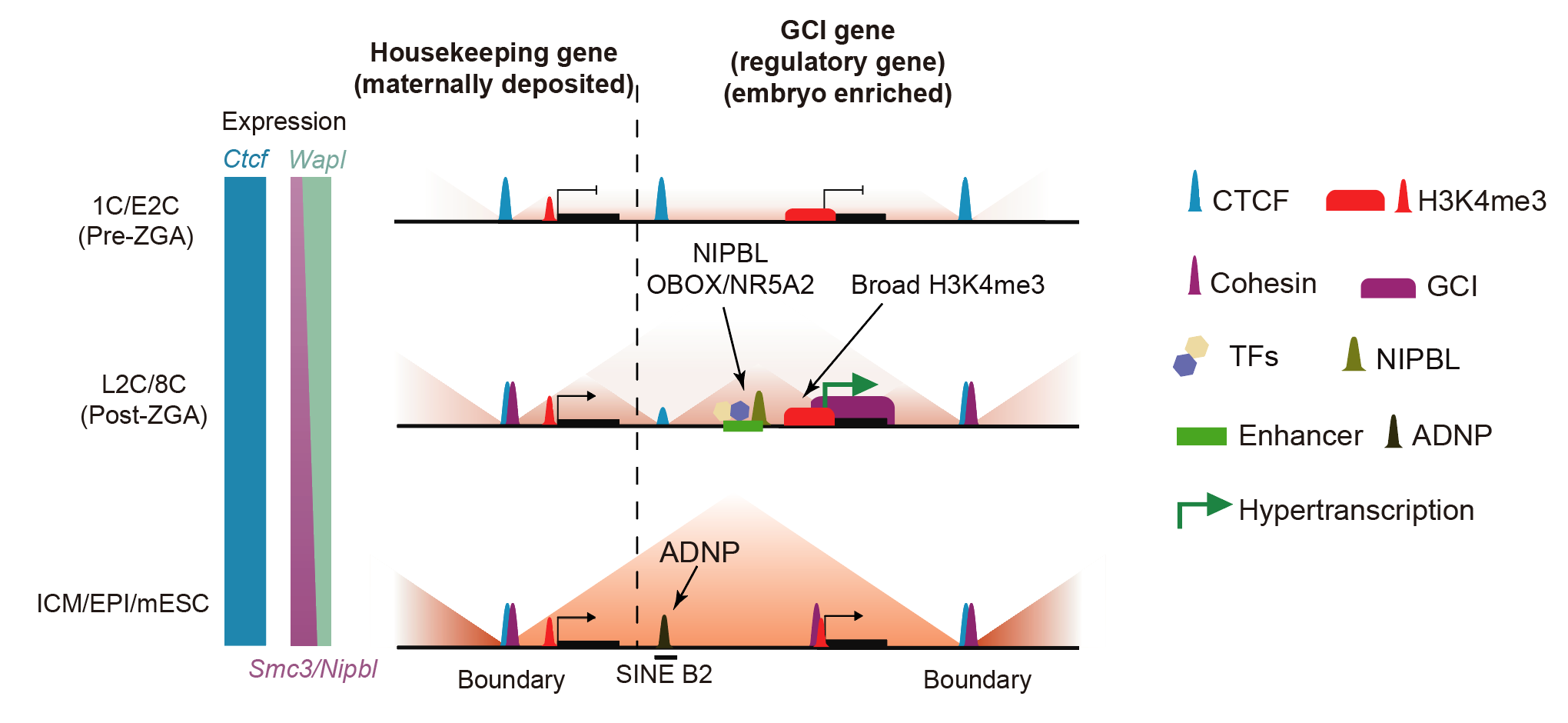 Figure 1. De novo establishment of chromatin architecture and its interplay with hypertranscription in early mouse embryos
Figure 1. De novo establishment of chromatin architecture and its interplay with hypertranscription in early mouse embryos
<関連情報>
- https://www.tsinghua.edu.cn/en/info/1245/14481.htm
- https://www.nature.com/articles/s41586-025-09400-5
クロマチン構造の確立は胚の過剰転写と相互作用する Establishment of chromatin architecture interplays with embryo hypertranscription
Guang Yu,Kai Xu,Weikun Xia,Ke Zhang,Qianhua Xu,Lijia Li,Zili Lin,Ling Liu,Bofeng Liu,Zhenhai Du,Xia Chen,Qiang Fan,Fangnong Lai,Wenying Wang,Lijuan Wang,Feng Kong,Chao Wang,Haiqiang Dai,Huili Wang & Wei Xie
Nature Published:13 August 2025
DOI:https://doi.org/10.1038/s41586-025-09400-5
Abstract
After fertilization, early embryos undergo dissolution of conventional chromatin organization, including topologically associating domains (TADs)1,2. Zygotic genome activation then commences amid unusually slow de novo establishment of three-dimensional chromatin architecture2. How chromatin organization is established and how it interplays with transcription in early mammalian embryos remain elusive. Here we show that CTCF occupies chromatin throughout mouse early development. By contrast, cohesin poorly binds chromatin in one-cell embryos, coinciding with TAD dissolution. Cohesin binding then progressively increases from two- to eight-cell embryos, accompanying TAD establishment. Unexpectedly, strong ‘genic cohesin islands’ (GCIs) emerge across gene bodies of active genes in this period. GCI genes enrich for cell identity and regulatory genes, display broad H3K4me3 at promoters, and exhibit strong binding of transcription factors and the cohesin loader NIPBL at nearby enhancers. We show that transcription is hyperactive in two- to eight-cell embryos and is required for GCI formation. Conversely, induced transcription can also create GCIs. Finally, GCIs can function as insulation boundaries and form contact domains with nearby CTCF sites, enhancing both the transcription levels and stability of GCI genes. These data reveal a hypertranscription state in early embryos that both shapes and is fostered by the three-dimensional genome organization, revealing an intimate interplay between chromatin structure and transcription.


