2025-09-16 ロックフェラー大学
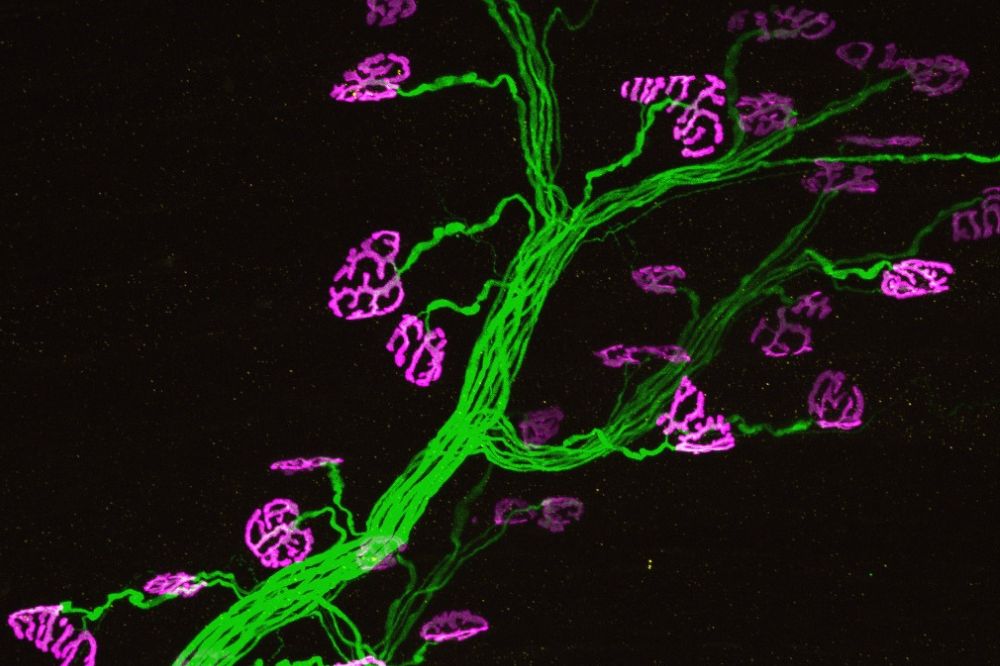
A motor neuron (with green axons and magenta axon terminals) restored by a PI31therapy. (Steller lab)
<関連情報>
- https://www.rockefeller.edu/news/38266-increasing-the-level-of-the-protein-pi31-demonstrates-neuroprotective-effects-in-mice/
- https://www.pnas.org/doi/10.1073/pnas.2511899122
PI31発現は若年性パーキンソン症候群マウスモデルにおいて神経保護作用を示す PI31 expression is neuroprotective in a mouse model of early-onset parkinsonism
Jose A. Rodriguez, Adi Minis, Rasha Aref, +2 , and Hermann Steller
Proceedings of the National Academy of Sciences Published:September 16, 2025
DOI:https://doi.org/10.1073/pnas.2511899122
Significance
Mutations in FBXO7/PARK15 cause early-onset parkinsonism in human patients. Using both Drosophila and conditional mouse KO models we demonstrate that FBXO7 deficiency leads to PI31 destabilization, impaired synaptic proteasome transport, and tau hyperphosphorylation. Transgenic expression of PI31 in neurons prevents neurodegeneration, greatly improves motor performance and synaptic integrity. It also extends lifespan up to fourfold in full-body Fbxo7 knockout mice. These findings reveal that PI31 is a key neuroprotective factor acting downstream of FBXO7. They also suggest that stimulating local proteasome activity at synapses can suppress synaptic dysfunction in neurodegenerative diseases. Therefore, our current study provides both fundamental insights into synaptic protein homeostasis and a framework for developing therapies to treat early-onset parkinsonism and related disorders.
Abstract
Neurodegenerative diseases present one of the most significant global health challenges. These disorders are defined by the accumulation of abnormal protein aggregates that impair synaptic function and cause progressive neuronal degeneration. Therefore, stimulating protein clearance mechanisms may be neuro-protective. Variants in FBXO7/PARK15 cause Parkinsonian Pyramidal Syndrome, an early-onset parkinsonian neurodegenerative disorder in humans, and inactivation of this gene in mice recapitulates many phenotypes seen in patients. The proteasome regulator PI31 is a direct binding partner of Fbxo7 and promotes local protein degradation at synapses by mediating fast proteasome transport in neurites. PI31 protein levels are reduced when the function of Fbxo7 is impaired. Here we show that restoring PI31 levels in Fbxo7 mutant fly and mouse strains prevents neuronal degeneration and significantly improves neuronal function, health, and lifespan. Notably, Fbxo7 inactivation in mouse neurons causes hyperphosphorylation of tau, and this was suppressed by transgenic expression of PI31. Our results demonstrate that PI31 is a crucial biological target through which Fbxo7 deficiency drives pathology. Therefore, targeting the PI31-pathway may represent a promising therapeutic approach for treating neurodegenerative disorders.


