2025-10-17 成育医療研究センター
<関連情報>
- https://www.ncchd.go.jp/press/2025/1017.html
- https://www.ncchd.go.jp/press/2025/1017.pdf
- https://www.jacionline.org/article/S0091-6749(25)00976-5/fulltext
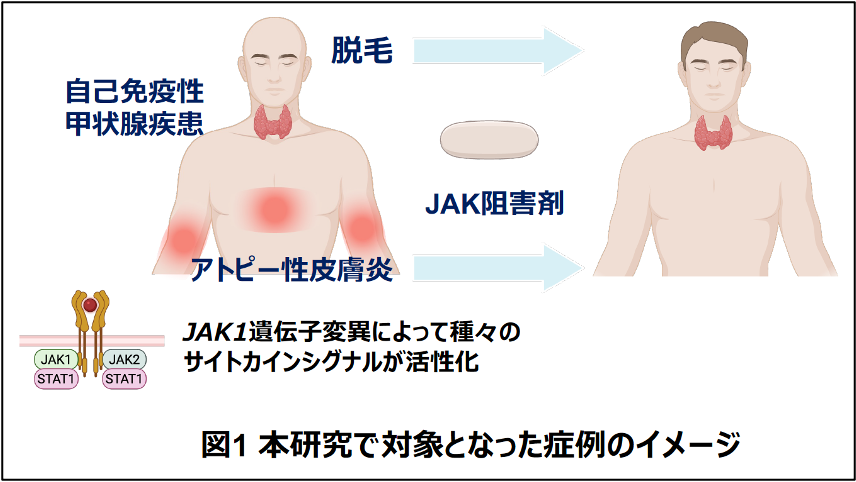
JAK1の機能獲得変異は円形脱毛症、アトピー性皮膚炎、自己免疫性甲状腺疾患を引き起こす AK1 gain-of-function variant causes alopecia areata, atopic dermatitis, and autoimmune thyroid disease
Satoshi Fujita, MD ∙ Shigenori Kabashima, MD, PhD ∙ Kumiko Yanagi, DDSc, PhD ∙ … ∙ Tadashi Kaname, MD, PhD ∙ Kenji Matsumoto, MD, PhD ∙ Hideaki Morita, MD, PhD
Journal of Allergy and Clinical Immunology Published:October 14, 2025
DOI:https://doi.org/10.1016/j.jaci.2025.09.012
To the Editor:
Recent advances in genomic analysis have revealed cases of severe or intractable allergic manifestations caused by inborn errors of immunity, including gain-of-function (GOF) or loss-of-function variants in the genes Janus kinase (JAK) and signal transducer and activator of transcription (STAT).1-3 Among them, 27 cases with JAK1 GOF have been reported, exhibiting autoimmune and/or inflammatory diseases, such as autoimmune thyroid diseases (AITD), sarcoidosis, inflammatory bowel disease, and atopic dermatitis (AD).4-8 This partly reflects the broad action of JAK1, which is involved in the downstream signaling of various cytokines, including IL-4, IFN-α, and IFN-γ. No reports have documented a JAK1 GOF variant causing alopecia areata (AA). Here we present a novel JAK1 GOF variant causing AA, intractable AD, and AITD by predominantly enhancing IFN-γ signaling.
The patient had severe widespread eczema at age 4 weeks and was diagnosed with AD at age 2 months. His itchy eczema was severe and refractory to conventional treatment with topical steroids, requiring repeated hospital admissions for intensive management. At age 3 years, he exhibited hair loss on his scalp and eyebrows, leading to a diagnosis of AA. Intermittent liquid nitrogen cryotherapy transiently improved his AA. However, by age 12 years, his hair loss had progressed to involve the entire scalp. He also exhibited short stature and failure to thrive, with no obvious causes such as growth hormone deficiency. Thyroid dysfunction, characterized by low serum levels of free triiodothyronine and free thyroxine, was incidentally detected at age 6 years, although he was asymptomatic. Notably, his levels of thyroid-stimulating hormone receptor antibody (27.5 IU/L), thyroglobulin antibody (534 IU/mL), and thyroid peroxidase antibody (514 IU/mL) were found to be simultaneously elevated, leading to a diagnosis of AITD and thyroid hormone replacement therapy. Interestingly, his father had a history of AA, intractable AD, and subcutaneous nodules that were initially diagnosed as Weber-Christian disease.
Owing to the possible hereditary nature of the patient’s diseases, trio-based whole-exome sequencing analysis was performed to identify their genetic etiology. After filtering based on population databases, 9 candidate variants remained, from among which we selected the JAK1 variant for functional validation on the basis of its relevance to JAK-STAT signaling pathways. Analysis revealed a novel heterozygous missense variant in JAK1 (NM_002227.4: c.2443C>T [p.Pro815Ser]) in the patient and his father but not in his mother. The variant was not registered in the gnomAD database (https://gnomad.broadinstitute.org/), jMORP database (https://jmorp.megabank.tohoku.ac.jp), or our in-house exome sequencing database (Fig 1, A). This variant was located in the pseudokinase domain of the JAK1 protein (Fig 1, A).