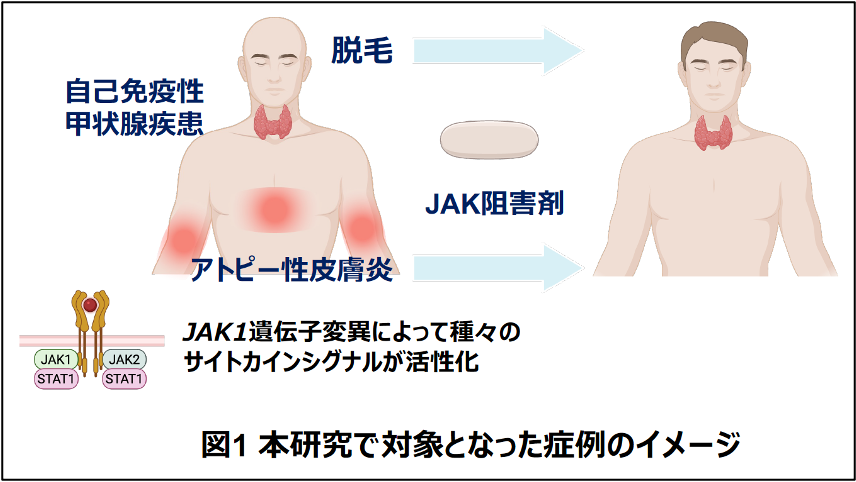
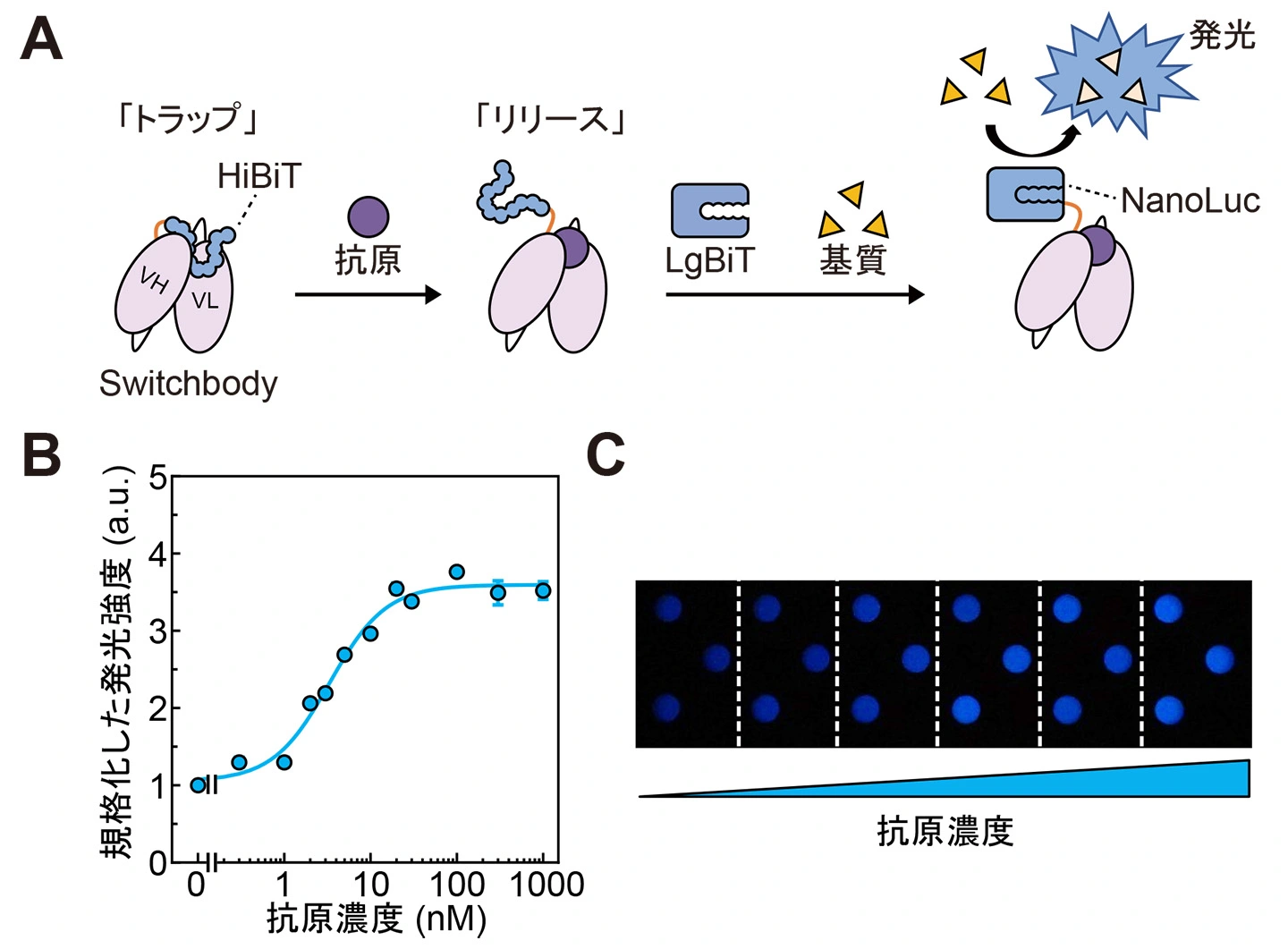
2025-10-16 京都大学
本研究の概要
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-10-16-0
- https://www.kyoto-u.ac.jp/sites/default/files/2025-10/web_2510_Takamiya-1771940e9f226a457430f11babb7690c.pdf
- https://www.mdpi.com/1422-0067/26/18/9102
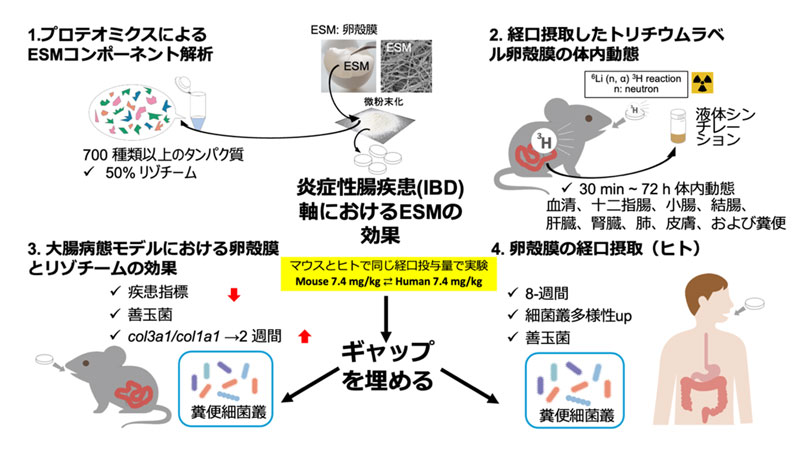
3H標識卵殻膜 を用いた薬物動態プロファイリングと卵殻膜およびリゾチームの経口補給がDSS誘発性大腸炎およびヒト腸内細菌叢に及ぼす影響 Pharmacokinetic Profiling Using 3H-Labeled Eggshell Membrane and Effects of Eggshell Membrane and Lysozyme Oral Supplementation on DSS-Induced Colitis and Human Gut Microbiota
Miho Shimizu,Wataru Sugai,Eri Ohto-Fujita,Aya Atomi,,Norio Nogawa,Koichi Takamiya,Hisao Yoshinaga,Yoshihide Asano,Takashi Yamashita,Shinichi Sato,Atsushi Enomoto,Nozomi Hatakeyama,Shunsuke Yasuda,Kazuya Tanaka,Tomoaki Atomi,Kenji Harada,Yukio Hasebe,Toshiyuki Watanabe and Yoriko Atomi
International Journal of Molecular Sciences Published: 18 September 2025
DOI:https://doi.org/10.3390/ijms26189102
Abstract
Eggshell membrane (ESM) is composed of approximately 90% protein. Our previous studies in healthy adults demonstrated that two months of daily ESM intake improved respiratory function, zigzag walking speed, and skin elasticity. The present study aims to address the knowledge gap regarding the in vivo effects of ESM in the context of inflammatory bowel disease (IBD). Proteomic analysis was performed on powdered ESM used as a dietary supplement. To investigate its pharmacokinetics in mice, tritium (3H)-labeled ESM was prepared using the 6Li(n,α)3H nuclear reaction. The therapeutic potential of ESM was further examined in a 2.0% dextran sulfate sodium (DSS)-induced murine model of IBD. In addition, fecal samples from both mice and healthy human subjects were analyzed using a modified terminal restriction fragment length polymorphism (T-RFLP) method. Lysozyme C (LYZ) was the most abundant protein (47%), followed by lysyl oxidase (12%) in ESM used in this study. 3H-ESM was mixed with MediGel, and orally administered to mice. Radioactivity levels were measured in blood, organs (duodenum, small intestine, large intestine, liver, kidney, lung, skin), and rectal feces at 0.5, 2, 5, 24, 48, and 72 h post-administration. Radioactivity in feces indicated excretion of undigested components, while systemic distribution suggested potential whole-body effects of ESM. Oral ESM and LYZ significantly alleviated body weight loss, diarrhea, and hematochezia in a DSS-induced murine model of IBD, leading to a significantly lower disease activity index on day 3 and showing a similar trend on day 5. Gut microbiota analysis showed increased Bacteroidales in the DSS group, while the ESM + DSS group maintained levels similar to the control. In humans, a double-blind, randomized controlled trial was conducted to evaluate the effects of ESM on gut microbiota in healthy adults. Participants received either ESM or placebo for 8 weeks. revealed a significant increase in alpha diversity at weeks 1 and 8 in the ESM group (p < 0.05), with between-group differences evident from week 1 (p < 0.01). ESM intake reduced Bacteroides and significantly increased Bifidobacterium and Lactobacillales at weeks 4 and 8. These findings suggest ESM supplementation promotes beneficial modulation of gut microbiota. These findings suggest that ESM, through its major protein components such as LYZ, may serve as a promising dietary intervention for maintaining intestinal health and mitigating inflammation in the context of IBD.