2025-10-31 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/new-insights-into-cell-biology-of-malaria-and-toxoplasmosis-parasites.html
- https://rupress.org/jcb/article/224/12/e202312109/278366/Tepsin-and-AP4-mediate-transport-from-the-trans
- https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3003415
- https://rupress.org/jcb/article-abstract/224/12/e202504062/278367/Vesicle-adaptors-in-malaria-parasites-show
テプシンとAP4はトキソプラズマにおいてトランスゴルジ体から植物様液胞への輸送を媒介する Tepsin and AP4 mediate transport from the trans-Golgi to the plant-like vacuole in toxoplasma
Janessa Grech,Abhishek Prakash Shinde,Javier Periz,Mirko Singer,Simon Gras,Ignasi Forné,Andreas Klingl,Joel B. Dacks,Elena Jiménez-Ruiz,Markus Meissner
Journal of Cell Biology Published:October 13 2025
DOI:https://doi.org/10.1083/jcb.202312109
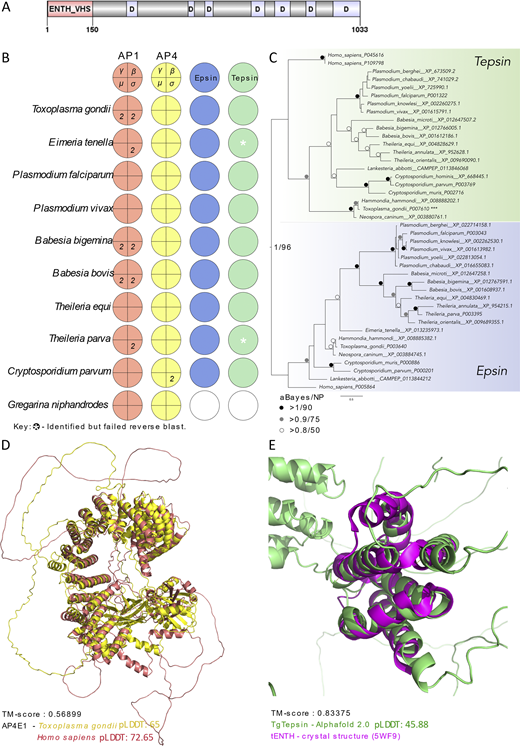
Apicomplexan parasites are obligate intracellular pathogens possessing unique organelles but lacking several components of the membrane trafficking machinery conserved in other eukaryotes. While some of these components have been lost during evolution, others remain undetectable by standard bioinformatics approaches. Using a conditional splitCas9 system in Toxoplasma gondii, we previously identified TGGT1_301410, a hypothetical gene conserved among apicomplexans, as a potential trafficking factor. Here, we show that TGGT1_301410 is a distant ortholog of T. gondii tepsin (TgTEP), localized to the trans-Golgi and functioning as an accessory protein of the adaptor protein complex 4 (AP4). We demonstrate that AP4-TgTEP is essential for the actin-dependent transport of vesicles to the plant-like vacuole (PLVAC) and Golgi organization. Notably, our findings reveal that, unlike in metazoans, the AP4 complex in T. gondii utilizes clathrin as a coat protein, a mechanism more closely aligned with that of plants. These results underscore a conserved yet functionally adapted vesicular transport system in Apicomplexa.
トキソプラズマ・ゴンディの細胞内複製中に細胞膜のリサイクルがリザーバー形成を促進する Plasma membrane recycling drives reservoir formation during Toxoplasma gondii intracellular replication
Julia von Knoerzer-Suckow ,Eva-Helena Aden ,Romuald Haase,Andreas Klingl,Ignasi Forné,Simon Gras
PLOS Biology Published: September 30, 2025
DOI:https://doi.org/10.1371/journal.pbio.3003415
Abstract
During intracellular development, apicomplexan parasites reside within a parasitophorous vacuole largely derived from the host plasma membrane (PM) and rendered nonfusogenic with the host endolysosomal system. Yet, the parasite is capable of protein uptake from the host cell via endocytosis, which occurs via a conserved structure, the micropore. Recently the composition of the micropore was characterized and its stability was shown to depend on the presence of the kelch-domain protein K13 which is also central to malarial drug-resistance to artemisinin. Interestingly, depletion of K13 also resulted in an impressive accumulation of PM attached to or between individual parasites, suggesting that the micropore plays a critical role in PM homoeostasis. Here, we characterized the dynamics and recycling of the PM in Toxoplasma gondii. In intracellular parasites, the PM is shared between individual parasites and undergoes a cycle of endocytosis and exocytosis during replication, similar to what has been previously demonstrated for extracellular parasites. This cycle appears to depend on Rab5b and MyoF. Interestingly, in contrast to Plasmodium falciparum, Rab5b is dispensable for the lytic cycle of T. gondii. During replication, parasites establish an extracellular plasma membrane reservoir (PMR) prior to daughter cell formation. The PMR is a dynamic membranous structure that varies in size and position throughout replication and disappears after daughter cell budding. Perturbation of the endo-exocytic balance disrupts PMR formation, leading to increased number and size of PMRs and, ultimately, to a complete loss of membrane organization directly linking endocytosis to the regulation of PMR formation.
マラリア原虫の小胞アダプターはタンパク質選別機構の保存性と柔軟性を示す Vesicle adaptors in malaria parasites show conservation and flexibility of protein sorting machinery
José Cubillán-Marín,Ulrike Fröhlke,Gala Ramón-Zamorano,Sheila Mainye,Joëlle Paolo Mesén-Ramírez,Guilherme B. Farias,Katharina Höhn,Tim-Wolf Gilberger,Richárd Bártfai,Tobias Spielmann
Journal of Cell biology Published:October 13 2025
DOI:https://doi.org/10.1083/jcb.202504062
Vesicle adaptors are critical for transport of proteins to the correct cellular destination. In malaria parasites general and specialized organelles depend on faithful protein transport to mediate host cell invasion and for intracellular survival. However, the role of adaptors in the parasite and the comparability of the sorting machinery with model organisms are unclear. Here, we show that AP-1, AP-3, and AP-4 are all important for parasite survival. AP-1 was needed for intracellular growth, biogenesis of specialized invasion organelles, and formation of invasive progeny, while AP-3 and AP-4 were both required for invasion into host cells. AP-1 acted through the multi-ligand receptor sortilin while AP-4 sorted multi-transmembrane proteins. Proxiomes from live cells revealed remarkable similarities of the configuration of the adaptor sorting machinery between the parasite and evolutionarily distant model organisms, but also unconventional features such as tepsin functioning with AP-1 and clathrin with AP-4. This work reveals unexpected exchangeability of key elements in otherwise surprisingly conserved adaptor sorting pathways.