2025-10-29 ノースウェスタン大学
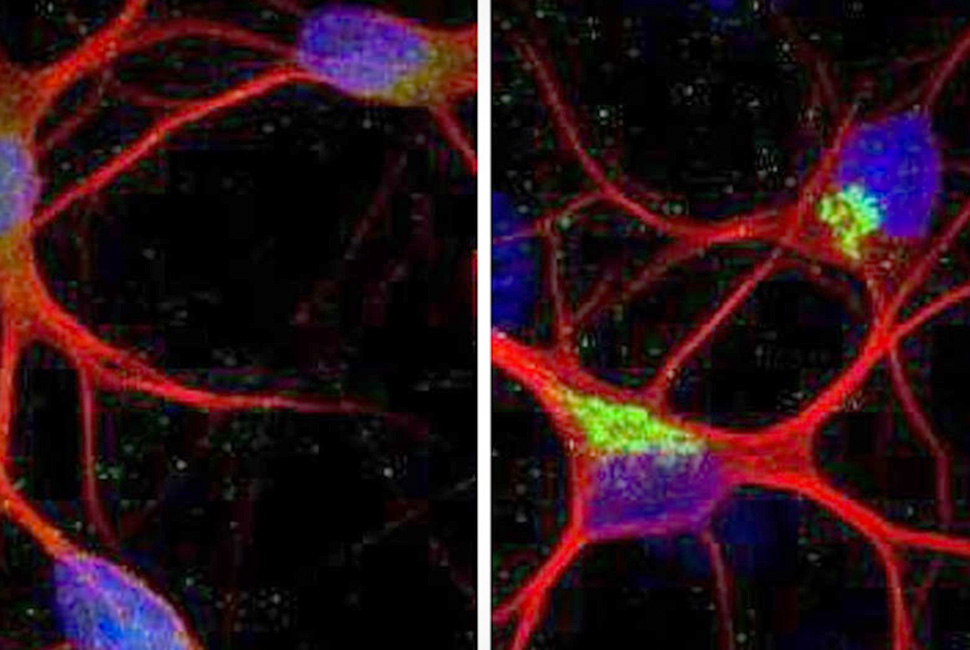
This side-by-side image shows control neurons from the study (left) compared to neurons from the study with the mis-spliced KCNQ2 gene.
<関連情報>
- https://news.northwestern.edu/stories/2025/10/new-clue-to-als-and-ftd-faulty-protein-disrupts-brains-brake-system
- https://www.nature.com/articles/s41593-025-02096-w
TDP-43依存性KCNQ2のミススプライシングはALS/FTDにおける内因性神経過興奮を引き起こす TDP-43-dependent mis-splicing of KCNQ2 triggers intrinsic neuronal hyperexcitability in ALS/FTD
Brian J. Joseph,Kelly A. Marshall,Peter Harley,Jacob R. Mann,Francesco Alessandrini,Carlos G. Vanoye,Wanhao Chi,Mercedes Prudencio,Dina Simkin,Tzu-Ting Kao,Reshma R. Desai,Matthew J. Keuss,Simone Barattucci,Matteo Zanovello,Puja R. Mehta,Jean-Marc DeKeyser,Francesco Limone,Jonathan Lee,Anna-Leigh Brown,Marcel F. Leyton-Jaimes,Leslie A. Nash,Irune Guerra San Juan,Eleonora Aronica,Brian J. Wainger,… Evangelos Kiskinis
Nature Neuroscience Published:31 October 2025
DOI:https://doi.org/10.1038/s41593-025-02096-w
Abstract
Motor neuron hyperexcitability is a broadly observed yet poorly understood feature of amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). Nuclear depletion and cytoplasmic aggregation of the RNA splicing protein TAR DNA-binding protein 43 (TDP-43) are observed in most ALS and FTD patients. Here we show that TDP-43 dysfunction causes mis-splicing of KCNQ2, which encodes a voltage-gated potassium channel (Kv7.2) that regulates neuronal excitability. Using iPSC-derived neurons and postmortem ALS/FTD brain and spinal cord tissue we find widespread, disease-specific and TDP-43-specific skipping of an exon encoding the KCNQ2 pore domain. The mis-spliced mRNA escapes degradation and is translated into a nonfunctional protein with severely reduced ion conductance that aggregates in the endoplasmic reticulum and causes intrinsic hyperexcitability in ALS neuronal models. This event, which correlates with higher phosphorylated TDP-43 levels and earlier age of disease onset in patients, can be rescued by splice-modulating antisense oligonucleotides that dampen hyperexcitability in induced pluripotent stem cell cortical neurons and spinal motor neurons with TDP-43 depletion. Our work reveals that nuclear TDP-43 maintains the fidelity of KCNQ2 expression and function and provides a mechanistic link between established excitability disruption in ALS/FTD patients and TDP-43 dysfunction.


