2025-11-10 ロードアイランド大学(URI)

The URI-built experimental model offers new way to look at TBI-related neurodegenerative disease.
<関連情報>
- https://www.uri.edu/news/2025/11/uri-team-creates-tabletop-blast-device-to-study-long-term-consequences-of-traumatic-brain-injury/
- https://www.cell.com/cell-reports-methods/fulltext/S2667-2375(25)00249-8
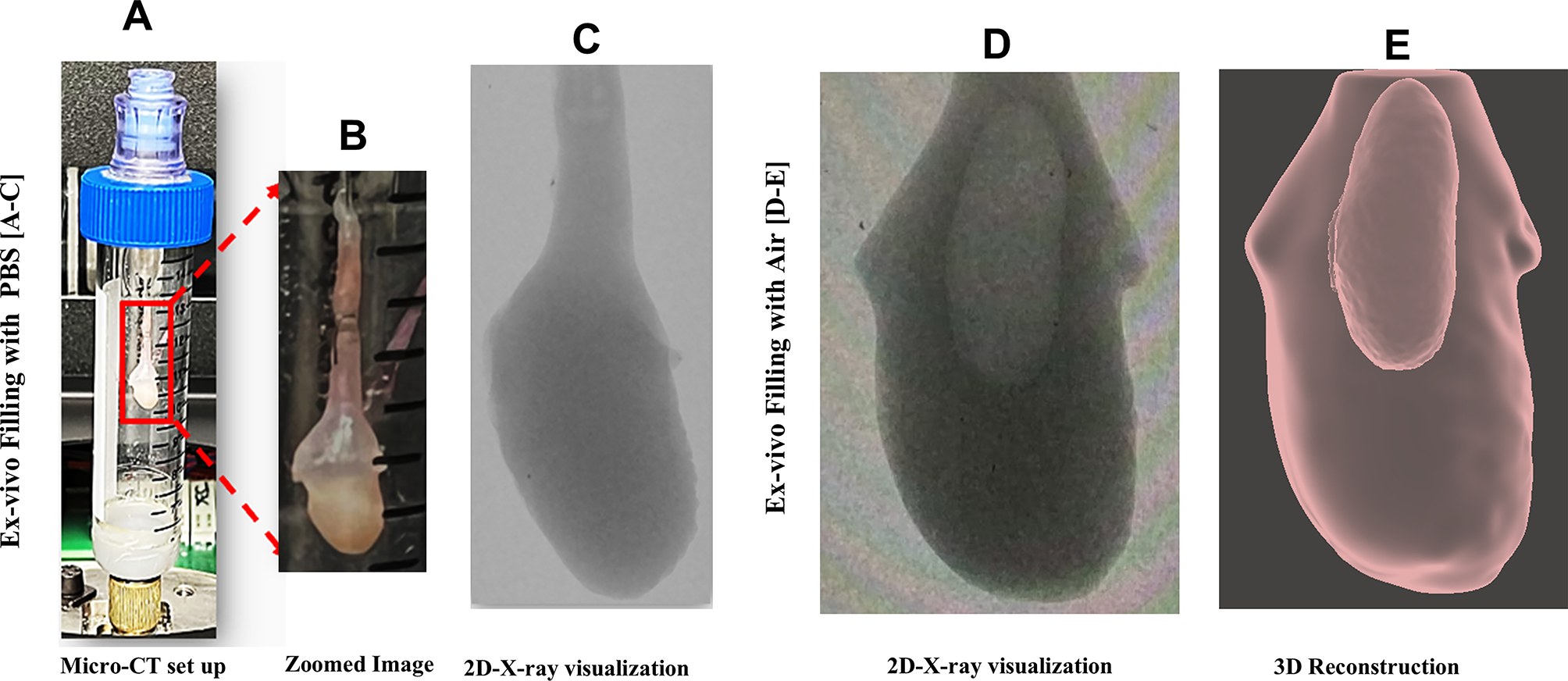
外傷性脳損傷が脳オルガノイドに及ぼす長期的な影響を研究するための卓上爆破装置 A tabletop blast device for the study of the long-term consequences of traumatic brain injury on brain organoids
Riccardo Sirtori ∙ Akash Pandey ∙ Arun Shukla ∙ Claudia Fallini
Cell Reports Methods Published:November 3, 2025
DOI:https://doi.org/10.1016/j.crmeth.2025.101213
Motivation
Modeling traumatic brain injury (TBI) in iPSC-derived brain organoids offers a valuable alternative to animal models, allowing for the study of human-specific processes affected by the injury in vitro. However, methodological variabilities and the need for expensive and specialized equipment have limited the adoption of this technology. Here, we developed a tabletop blast device capable of delivering tunable pressure waves to brain organoids in suspension, designed for easy implementation in standard biomedical laboratories.
Highlights
- To model TBI in vitro, we develop an easily implementable tabletop blast device
- Our device can deliver tunable pressure waves to human brain organoids
- Blast waves induce acute neurodegeneration and chronic dysfunction in neurons
Summary
Traumatic brain injury (TBI) is the leading environmental risk factor for neurodegenerative diseases, yet its molecular link to chronic neurodegeneration is unclear. While animal models of TBI are commonly used, emerging research suggests that induced pluripotent stem cell (iPSC)-derived brain organoids offer a promising human-specific alternative, particularly for studying processes like cryptic exon splicing. However, widespread use has been limited by methodological variability and the need for expensive and specialized equipment. To address these challenges, we developed a tabletop blast device capable of delivering highly reproducible pressure waves via a gravity-based pressure chamber. We validated the applicability of our approach by assessing the short- and long-term consequences of mechanical stress on brain organoids after pressure wave exposure. Our approach provides a controllable and reproducible method to apply complex pressure cycles on brain organoids, enabling broader accessibility for studying the mechanistic links between TBI and neurodegeneration in a human-relevant context.