2025-11-14 ロックフェラー大学
<関連情報>
- https://www.rockefeller.edu/news/38627-this-molecular-switch-helps-cancer-cells-survive-harsh-conditions/
- https://www.nature.com/articles/s41589-025-02035-7
MED1 IDR脱アセチル化はRNAポリメラーゼIIのリクルートメントを介してストレス応答遺伝子を制御する MED1 IDR deacetylation controls stress responsive genes through RNA Pol II recruitment
Ran Lin,Yan Mo,Douglas Barrows,Wenbin Mei,Takashi Onikubo,Jianfeng Sun,Zhiguo Zhang,Effie Apostolou,Sohail F. Tavazoie & Robert G. Roeder
Nature Chemical Biology Published:23 October 2025
DOI:https://doi.org/10.1038/s41589-025-02035-7
Abstract
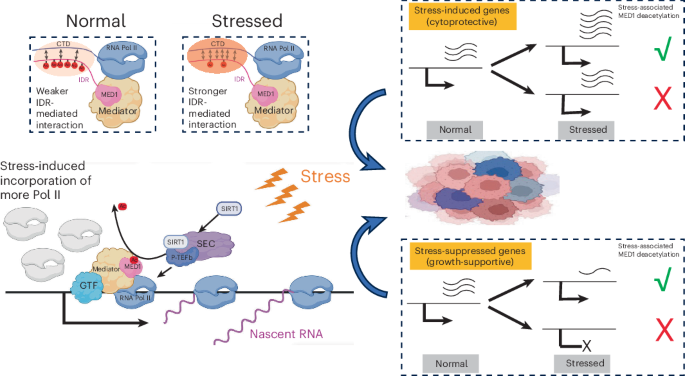
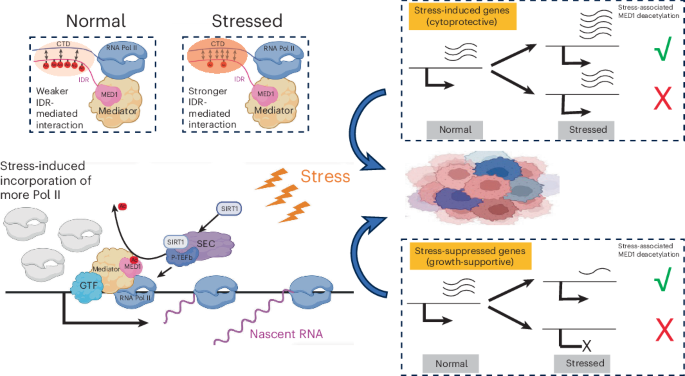
Cells fine-tune gene expression in response to cellular stress, a process critical for tumorigenesis. However, mechanisms governing stress-responsive transcription remain incompletely understood. This study shows that the MED1 subunit of the Mediator coactivator complex is acetylated in its intrinsically disordered region (IDR). Under stress, SIRT1 associates with the super elongation complex to deacetylate MED1 in promoter-proximal regions. The deacetylated (or acetylation-defective mutant) MED1 amplified stress-activated cytoprotective genes and rescued stress-suppressed growth-supportive genes in estrogen-receptor-positive breast cancer (ER+ BC) cells. Mechanistically, deacetylated MED1 promotes chromatin incorporation of RNA polymerase II (Pol II) through IDR-mediated interactions. Functionally, ER+ BC cells with deacetylated MED1 exhibit faster growth and enhanced stress resistance in culture and in an orthotopic mouse model. These findings advance our understanding of Pol II regulation under cellular stress and potentially suggest therapeutic strategies targeting oncogenic transcription driven by MED1 and Mediator.