2025-11-20 東京大学
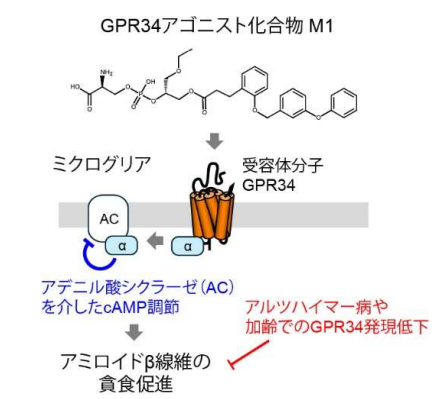
GPR34 活性化によるミクログリアのアミロイド β 貪食促進
<関連情報>
- https://www.u-tokyo.ac.jp/focus/ja/press/z0111_00092.html
- https://www.u-tokyo.ac.jp/content/400274731.pdf
- https://alzres.biomedcentral.com/articles/10.1186/s13195-025-01891-8
GPR34の選択的アゴニストはミクログリア細胞によるアミロイドβ線維の取り込みと除去を促進する Selective agonism of GPR34 stimulates microglial uptake and clearance of amyloid β fibrils
Hayato Etani,Sho Takatori,Wenbo Wang,Jumpei Omi,Yusuke Amiya,Aika Akahori,Hirotaka Watanabe,Iki Sonn,Hideyuki Okano,Norikazu Hara,Mai Hasegawa,Akinori Miyashita,Masataka Kikuchi,Takeshi Ikeuchi,Maho Morishima,Yuko Saito,Shigeo Murayama,Takashi Saito,Takaomi C. Saido,Toshiyuki Takai,Tomohiko Ohwada,Junken Aoki & Taisuke Tomita
Alzheimer’s Research & Therapy Published:20 November 2025
DOI:https://doi.org/10.1186/s13195-025-01891-8
Abstract
Background
Microglia play a crucial role in brain homeostasis through phagocytosis of amyloid-β (Aβ) fibrils, a hallmark of Alzheimer disease (AD) pathology. The balance between Aβ production and clearance is critical for AD pathogenesis, with impaired clearance mechanisms potentially contributing to disease progression. G-protein coupled receptor 34 (GPR34), a microglia-enriched Gi/o-coupled receptor, is highly expressed in homeostatic microglia and may regulate phagocytic functions, yet its role in Aβ clearance remains poorly understood.
Methods
Using flow cytometry-based assays, we investigated the effect of a selective GPR34 agonist (M1) on Aβ uptake in mouse primary microglia and human induced pluripotent stem cell-derived microglia. We evaluated uptake specificity across different Aβ species and phagocytic substrates, and measured intracellular cyclic adenosine monophosphate (cAMP) levels to determine the signaling mechanism. We performed in vivo studies using human amyloid precursor protein knock-in mice with intrahippocampal M1 injections. Additionally, we analyzed GPR34 expression in Japanese AD patient brain samples using single-nucleus RNA sequencing and examined age-dependent expression changes across multiple datasets.
Results
M1 specifically enhanced uptake of Aβ fibrils through reduction of intracellular cAMP levels, without affecting monomeric or oligomeric Aβ internalization. Gpr34 knockdown experiments confirmed GPR34 as the molecular target of M1. An intrahippocampal injection of M1 significantly increased microglial Aβ uptake in vivo, an effect that required functional TREM2 signaling. GPR34 expression was significantly reduced in microglia from AD patients and showed age-dependent decline in both humans and mice.
Conclusions
Our findings identify GPR34 as a promising therapeutic target for enhancing microglial Aβ clearance and highlight the potential of GPR34 agonists for AD treatment. The age-dependent decline in GPR34 expression may contribute to reduced Aβ clearance efficiency in aging brains, exacerbating amyloid accumulation. Pharmacological activation of GPR34 represents a novel strategy to counteract impaired Aβ clearance in both aging and AD brains, potentially modifying disease progression through enhancement of microglial phagocytic function.