2025-12-24 韓国基礎科学研究院(IBS)
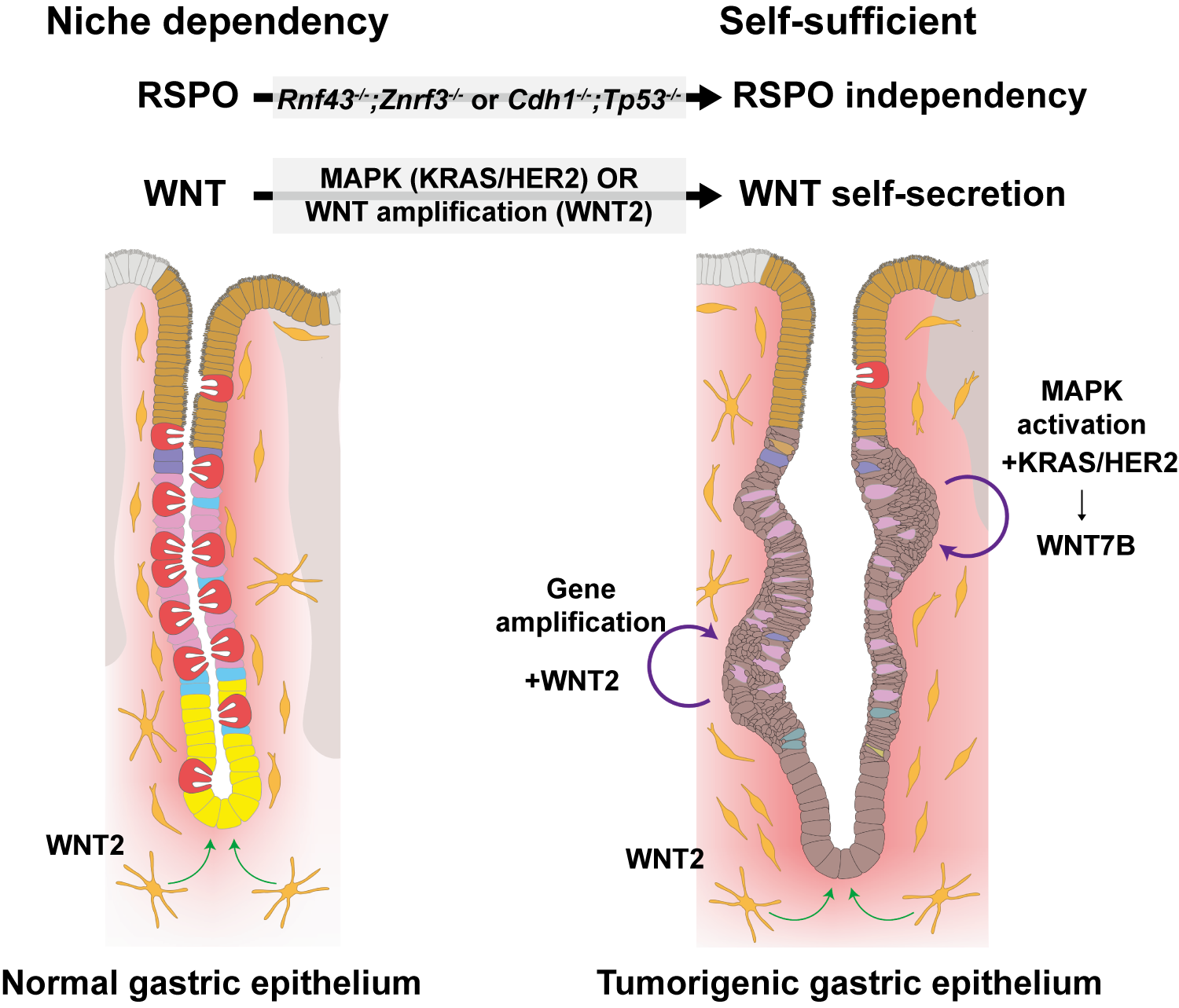
Figure 1. Differences in Growth Signal Regulation between Normal and Tumorigenic Gastric Epithelium
Left: In normal gastric tissue, gastric epithelial cells rely on growth signals provided by the surrounding microenvironment. In particular, WNT signals — such as WNT2 secreted by neighboring cells — activate WNT signaling in gastric epithelial cells, allowing gastric stem cells to survive, self-renew, and continuously regenerate the stomach lining. Without this external WNT supply, normal gastric stem cells cannot maintain their function.
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do?nttId=26456&pageIndex=1&searchCnd=&searchWrd=
- https://link.springer.com/article/10.1186/s12943-025-02543-z
上皮細胞からのWNT分泌が胃癌の進行過程におけるニッチからの脱出を促進する Epithelial WNT secretion drives niche escape of developing gastric cancer
Jaehun Lee,Soomin Kim,Youngchul Oh,Stephan R. Jahn,Jihoon Kim,Yeongjun Kim,Tim Schmäche,Sang-Min Kim,Isaree Teriyapirom,Thomas Groß,Ohbin Kwon,Jungmin Kim,Somi Kim,Anne-Marlen Ada,Andrea Català-Bordes,Youngwon Cho,Jinho Kim,Amanda Andersson-Rolf,Sebastian R. Merker,Joo Yeon Lim,Ji-Yeon Park,Thomas M. Klompstra,Ki-Jun Yoon,Dae-Sik Lim,… Ji-Hyun Lee
Molecular Cancer Published:16 December 2025
DOI:https://doi.org/10.1186/s12943-025-02543-z
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
Abstract
Background
WNT signaling plays a key role in maintaining the gastric epithelium and promoting tumorigenesis. However, how gastric tumors achieve WNT niche independence remains unclear, as mutations on APC or CTNNB1—common mechanisms of ligand-independent WNT activation in colorectal cancer—are infrequent in gastric cancer. Understanding how WNT self-sufficiency is acquired in the stomach is therefore critical.
Methods
We analyzed mouse gastric organoids harboring oncogenic KRASG12D with or without RNF43/ZNRF3 (RZ) or CDH1/TP53 (CP) mutations, along with corresponding in vivo mouse models. Niche independence was assessed through growth factor withdrawal, Porcupine and pathway-specific inhibitor treatments, and WNT rescue assays. We performed single-nucleus multiome sequencing (RNA + ATAC) to investigate transcriptional and chromatin dynamics. Findings from mouse models were validated using patient-derived gastric cancer organoids, and pan-cancer cell line datasets were analyzed to evaluate clinical and cross-tissue relevance.
Results
Gastric fibroblasts secreted canonical WNT2B to maintain the homeostatic gastric epithelium. Upon KRAS activation, epithelial cells were reprogrammed to secrete WNT ligands independently of additional mutations. Single-nucleus multiome analysis revealed that KRAS-driven MAPK signaling opened SMAD2/3-bound enhancers at the WNT7B locus, leading to the emergence of WNT7B-expressing subpopulations. Inhibition of SMAD2/3 phosphorylation suppressed both organoid growth and WNT7B transcription, whereas exogenous WNT restored organoid proliferation. Patient-derived organoids with HER2 amplification, KRAS amplification, or WNT2 copy-number gain exhibited Porcupine inhibitor-sensitive growth, indicating dependence on WNT secretion from the organoids. Analysis of public transcriptomic datasets further demonstrated that the KRAS–MAPK–WNT7B axis is conserved across other cancer types, including lung cancer.
Conclusions
Gastric tumors can bypass niche dependence by acquiring KRAS–MAPK–SMAD2/3-driven epithelial WNT secretion. Targeting this axis—through MAPK inhibition, SMAD2/3 blockade, or suppression of WNT secretion—may represent a therapeutic vulnerability in gastric cancer and other KRAS-high malignancies.