2026-01-12 ゲーテ大学
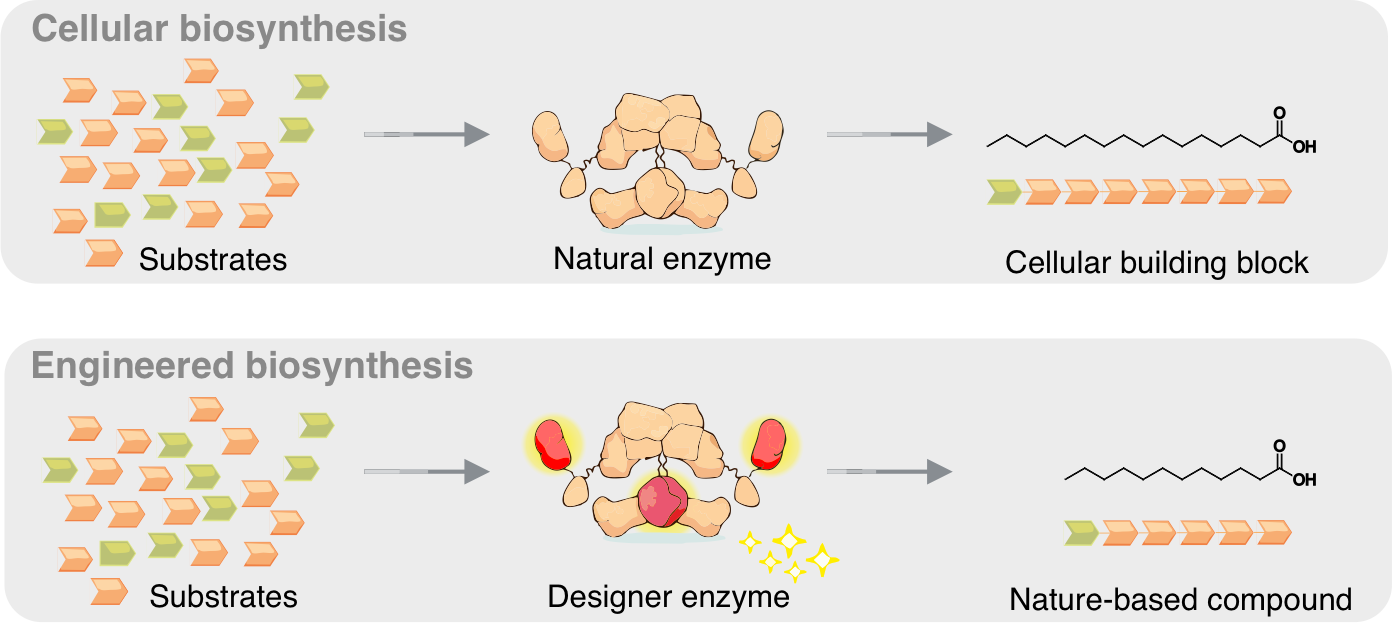
Schematic representation of biosynthesis in a cell (top) and in the laboratory (bottom). The designer enzyme shortens the chain length of the fatty acid (Image: Felix Lehmann & Martin Grininger/Goethe University).
<関連情報>
- https://aktuelles.uni-frankfurt.de/english/from-palm-oil-to-designer-enzymes-frankfurt-researchers-reprogram-yeast-cells/
- https://www.nature.com/articles/s41589-025-02105-w
- https://onlinelibrary.wiley.com/doi/10.1002/anie.202511726
後生動物の脂肪酸合成酵素を改変し、鎖長を制御して酵母に適用 Engineering metazoan fatty acid synthase to control chain length applied in yeast
Damian L. Ludig,Xiaoxin Zhai,Alexander Rittner,Christian Gusenda,Maximilian Heinz,Svenja Berlage,Ning Gao,Adrian J. Jervis,Yongjin J. Zhou &Martin Grininger
Nature Chemical biology Published:07 January 2026
DOI:https://doi.org/10.1038/s41589-025-02105-w
Abstract
Metazoan fatty acid (FA) synthases (mFASs) facilitate the de novo synthesis of C16- and C18-FAs through iterative extensions within the FA cycle and hydrolytic release. Here we re-engineer mFAS to fine-tune the interplay between FA extension and FA hydrolytic release for the targeted production of short- and medium-chain fatty acids. Single amino acid exchanges in the ketosynthase domain can redirect FA product profiles from predominantly C8 (G113W) to C8/C10 (G113F) and C12/C14 (G113M). Integration of a thioreductase domain enables the production of medium-chain fatty aldehydes and alcohols. We apply our approach for controlling chain length in FA biosynthesis to the microbial production of C10- and C12-FAs, translate it into a yeast cell factory and achieve C10/C12-FAs titers of 674 mg l-1 and 67% purity of total free FAs. Our work demonstrates a modular platform for programmable FA synthesis and paves the way toward sustainable bioproduction of valuable oleochemicals.
2-ピロンポリケチドの区画化生産のためのマウス脂肪酸合成酵素由来の多酵素の設計 Design of a Multienzyme Derived from Mouse Fatty Acid Synthase for the Compartmentalized Production of 2-Pyrone Polyketides
Felix Lehmann, Nadja Joachim, Carolin Parthun, Prof. Martin Grininger
Angewandte Chemie International Edition Published: 17 November 2025
DOI:https://doi.org/10.1002/anie.202511726
Abstract
Compartmentalizing biosynthetic pathways is a key objective in protein engineering, particularly in synthetic biology and metabolic engineering. It can improve catalytic efficiency, stabilize reactive intermediates, reduce by-product formation, and, beyond these advantages, enable synthetic complexity by coordinating multistep pathways. In this study, we established a chemoenzymatic platform for producing 2-pyrones—specifically styrylpyrones and hispidin—within a multienzyme based on a non-reducing (nr) variant of the murine fatty acid synthase (FAS), which naturally produces palmitic acid. By introducing two amino acid substitutions in the ketosynthase (KS) domain, we enhanced the nrFAS-mediated synthesis of styrylpyrones from non-native substrates, including halogenated derivatives. The engineered enzyme exhibited a 66-fold increase in activity compared to the non-mutated nrFAS, surpassing the styrylpyrone synthase of the kavalactone pathway in Piper methysticum. Additionally, we integrated a 4-coumarate ligase (4CL1) loading module into the compartment using the SpyTag/SpyCatcher system, enabling the activation and direct transfer of cinnamic acid derivatives to the nrFAS. The resulting styrylpyrones are direct precursors of pharmaceutically active kavalactones, while hispidin serves as the precursor of fungal bioluminescence.


