2026-01-09 ワシントン大学セントルイス校
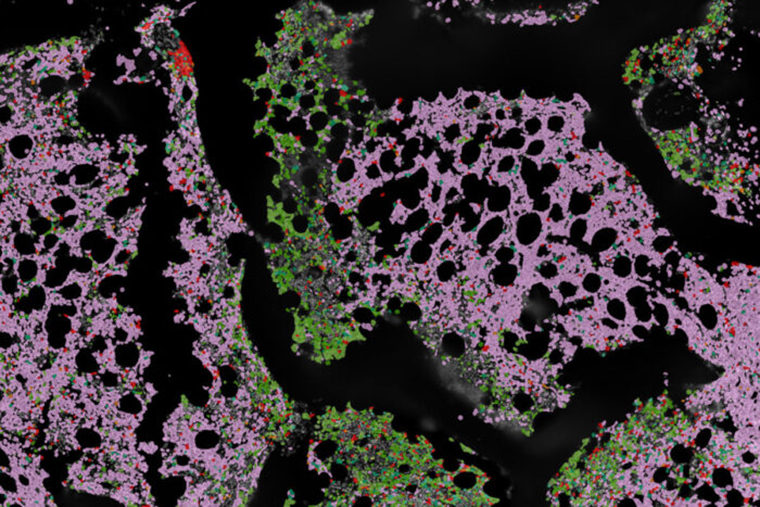
Researchers at WashU Medicine and their collaborators have created an immune cell atlas of multiple myeloma, a cancer of the bone marrow. The new resource could improve prognosis and guide development of new immunotherapies. Shown is a sample of bone marrow from a patient with multiple myeloma, indicated by an overabundance of plasma cells (pink) compared with normal bone marrow (green). T cells are in red. (Image: Julia Wang)
<関連情報>
- https://source.washu.edu/2026/01/inflammatory-immune-cells-predict-survival-relapse-in-multiple-myeloma/
- https://medicine.washu.edu/news/inflammatory-immune-cells-predict-survival-relapse-in-multiple-myeloma/
- https://www.nature.com/articles/s43018-025-01072-4
単一細胞アトラスは、多発性骨髄腫の転帰に関連する骨髄免疫微小環境の調節不全を特徴づける A single-cell atlas characterizes dysregulation of the bone marrow immune microenvironment associated with outcomes in multiple myeloma
William C. Pilcher,Lijun Yao,Edgar Gonzalez-Kozlova,Yered Pita-Juarez,Dimitra Karagkouni,Chaitanya R. Acharya,Marina E. Michaud,Mark Hamilton,Shivani Nanda,Yizhe Song,Kazuhito Sato,Julia T. Wang,Sarthak Satpathy,Yuling Ma,Jessica Schulman,Darwin D’Souza,Reyka G. Jayasinghe,Denis Ohlstrom,Katherine E. Ferguson,Giulia Cheloni,Mojtaba Bakhtiari,Nick Pabustan,Kai Nie,Jennifer A. Foltz,Immune Atlas Consortium,… Manoj Bhasin
Nature Cancer Published:09 January 2026
DOI:https://doi.org/10.1038/s43018-025-01072-4
Abstract
Multiple myeloma (MM) remains incurable despite advances in treatment options. Although tumor subtypes and specific DNA abnormalities are linked to worse prognosis, the impact of immune dysfunction on disease emergence and/or treatment sensitivity remains unclear. We developed an Immune Atlas of MM by generating profiles of 1,397,272 single cells from the bone marrow (BM) of 337 newly diagnosed participants and characterized immune and hematopoietic cell populations. Cytogenetic risk-based analysis revealed heterogeneous associations with T cells of BM, with 17p13 deletion showing distinct enrichment of a type 1 interferon signature. The disease progression-based analysis revealed the presence of a proinflammatory immune senescence-associated secretory phenotype in rapidly progressing participants. Furthermore, signaling analyses suggested active intercellular communication involving a proliferation-inducing ligand and B cell maturation antigen, potentially promoting tumor growth and survival. Lastly, using independent discovery and validation cohorts, we demonstrated that integrating immune cell signatures with known tumor cytogenetics and individual characteristics significantly improves stratification for the prediction of survival.