2026-01-16 ミュンヘン大学(LMU)
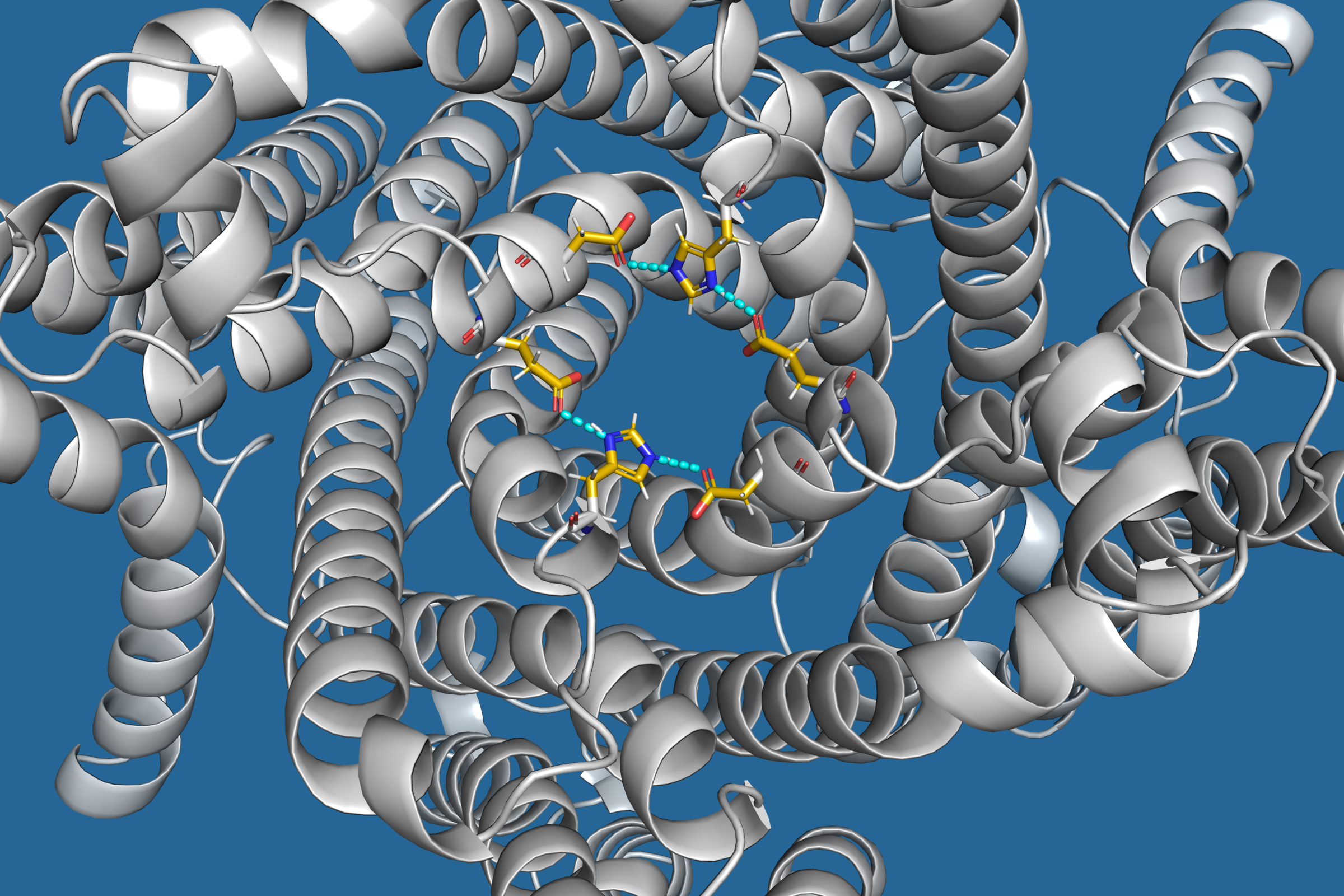
TMEM175 from the perspective of the lysosomal interior. © O. Rauh
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/parkinsons-ion-channel-as-promising-avenue-for-new-drugs-5df049ce.html
- https://www.pnas.org/doi/10.1073/pnas.2503909123
リソソーム陽イオンチャネルTMEM175のプロトン選択的伝導とゲーティング Proton-selective conductance and gating of the lysosomal cation channel TMEM175
Tobias Schulze, Timon Sprave, Carolin Groebe, +9 , and Oliver Rauh
Proceedings of the National Academy of Sciences Published:January 14, 2026
DOI:https://doi.org/10.1073/pnas.2503909123
Significance
Malfunction of the lysosomal ion channel TMEM175 disrupts luminal pH homeostasis and has been linked to neurodegenerative disorders, such as Parkinson’s disease. The channel’s principal ion selectivity remains a subject of ongoing debate, with conflicting evidence supporting K+ or H+ as the dominant permeant species. To investigate the channel’s selectivity and pH dependence, we analyzed TMEM175-mediated currents in response to changes in luminal pH. Electrophysiological recordings revealed that luminal acidification activates a H+-conductance, leading to rapid collapse of the pH-gradient. Integrating experimental and computational approaches, we identified H57 as a key residue regulating TMEM175-mediated H+ flux. Presented findings deepen our understanding of human TMEM175 structure–function and broaden the possibilities for developing therapeutic approaches for the treatment of TMEM175-associated neurodegenerative diseases.
Abstract
The lysosomal cation channel TMEM175 plays a key role in luminal pH homeostasis and lysosome function, with aberrant activity linked to Parkinson’s disease. Although initially described as a K+-selective channel, TMEM175 exhibits substantial H+ permeability. Here, we dissect complex changes affecting human TMEM175 conductance and ionic properties of TMEM175-mediated current in response to pH shifts on the luminal side of the protein. A drop in pH from 7.4 to 4.7 on the side equivalent to the lysosomal lumen triggers a sustained increase in TMEM175-mediated inward and outward currents, which is accompanied by a transient shift in the reversal potential (Erev) toward the theoretical equilibrium voltage for H+, yet remaining ~100 mV below the expected value even in the absence of K+. This discrepancy, along with low sensitivity of Erev to the concentration gradient for K+, supports a model in which TMEM175-mediated H+ flux rapidly collapses the lysosomal pH-gradient. Molecular dynamics simulations identify H57 as a key residue on the luminal side of the open channel, which forms intra- and intersubunit salt bridges with D279 and E282. Supporting the functional importance of these interactions, the TMEM175 mutant H57Y displayed reduced H+– and K+-conductance and a reduced H+/K+ selectivity in whole-cell and lysosomal electrophysiological analyses. Our findings contribute to a better understanding of TMEM175’s complex electrophysiological properties, thereby expanding the possibilities of understanding the channel’s function in lysosomal physiology and pathophysiology.


