2026-01-16 パシフィック・ノースウェスト国立研究所(PNNL)
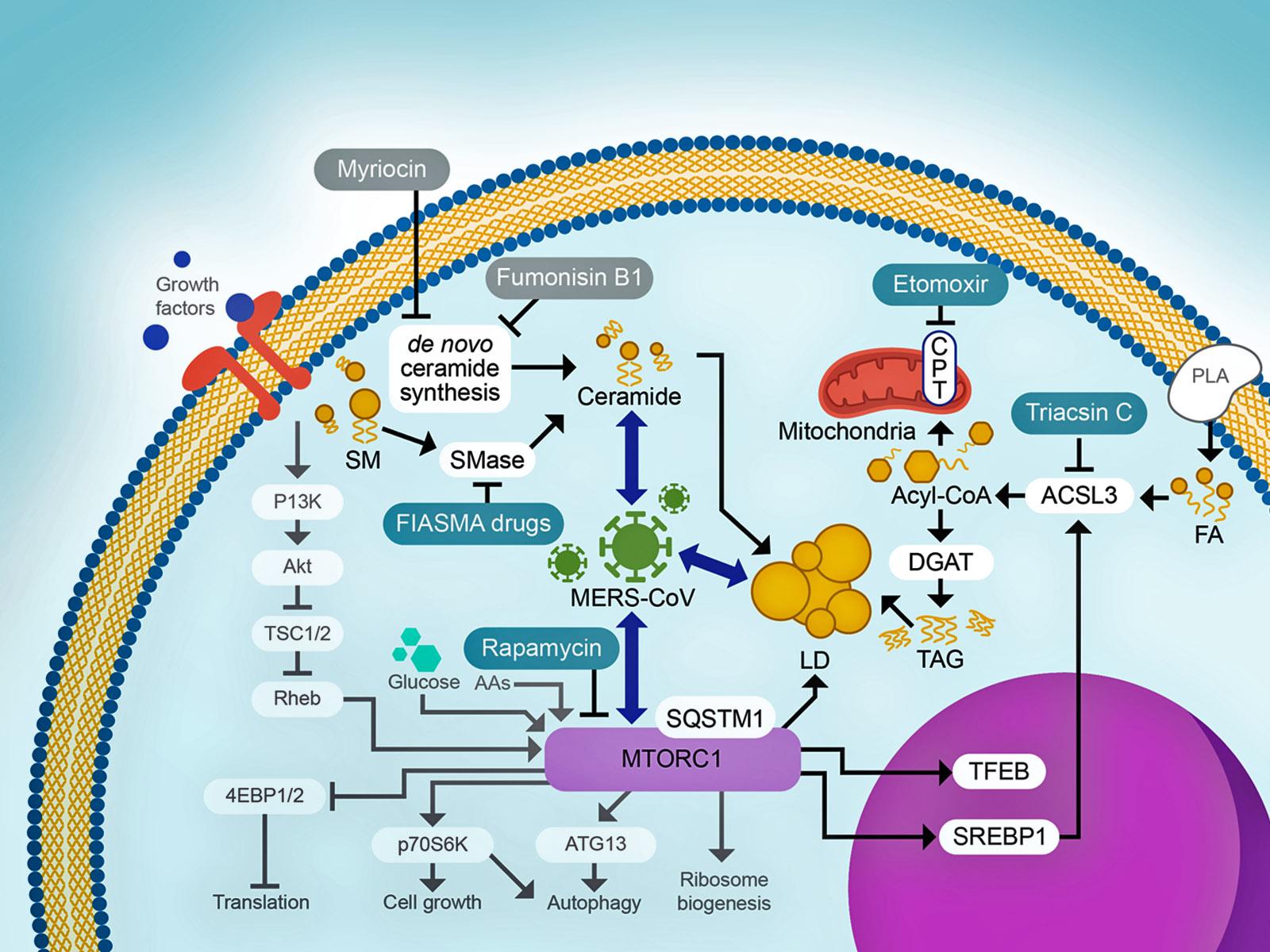
Model of lipid related cell signaling during MERS-CoV infection.(Illustration by Hugh Mitchell and Stephanie King | Pacific Northwest National Laboratory)
<関連情報>
- https://www.pnnl.gov/publications/selective-targeting-triglycerides-and-ceramides-prevents-mers-cov-replication
- https://journals.asm.org/eprint/B5JQZT8SWCCFYXCEYXS8/full
トリアシルグリセリドとセラミドの増加はMERS-CoVの複製に重要である Increased triacylglyceride and ceramide levels are key for MERS-CoV replication
Hugh D. Mitchell, Jennifer Kyle, Kristin Engbrecht, Madelyn Berger, Kristie L. Oxford, Amy C. Sims
mSphere Published:15 January 2026
DOI:https://doi.org/10.1128/msphere.00523-25
ABSTRACT
Emerging viruses remain a threat to human health; however, many aspects of their infection cycle are still poorly understood. Host lipid structures and abundances are observed to be significantly altered during infection, and the mechanisms regulating lipid synthesis and modification remain largely unknown. In this work, we analyzed a large multi-omic data set from three Middle East respiratory syndrome coronavirus (MERS-CoV)-infected primary human lung cell types, all derived from three distinct donors to investigate the changes in lipid species during infection. Analysis of lipidomics data identified perturbations of various lipid classes, and we hypothesized and confirmed that MERS-CoV infection orchestrates an increase in ceramide via sphingomyelinase pathways required for infection. We also identified a minor subset of proteins with lipid-related functions with increased differential expression among a striking majority of lipid-related proteins with decreased differential expression. The most prominent of these is ACSL3, a long-chain acyl-CoA synthetase that is key for the synthesis of triacylglycerides and is associated with lipid droplet formation, an established feature of coronavirus-infected cells. Accordingly, the inhibition of acyl-CoA synthetase activity reduced MERS-CoV replication. These results suggest a model wherein coronaviruses perturb overall cellular metabolism to shift resources to the production of ceramides and triacylglycerides, particularly through acyl-CoA synthetase activity. Our findings suggest a strategy for targeting CoV replication through the inhibition of specific subsets of lipid metabolism.