2026-01-21 中国科学院(CAS)
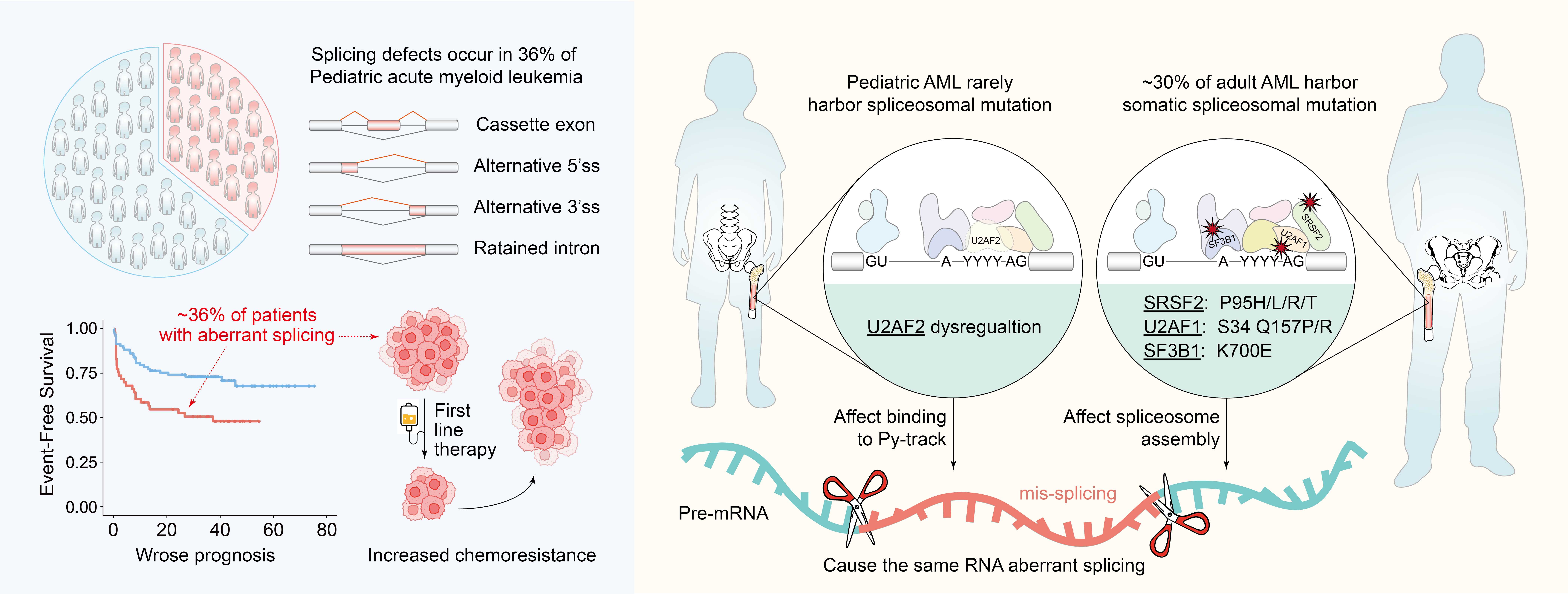
An overview of widespread splicing dysregulation in pediatric AML. (Image by Profs. WANG Qianfei and LIU Zhaoqi’s teams)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202601/t20260120_1146273.shtml
- https://www.sciencedirect.com/science/article/pii/S2666379125006159
配列依存性スプライシング異常が小児AMLの治療抵抗性を引き起こす Sequence-dependent splicing dysregulation drives therapy resistance in pediatric AML
Yue Huang, Peifang Xiao, Lei Qin, Li Gao, Yang Zhao, Jingru Sui, Wenting Hu, Lei Zhou, Nan Han, Xuan Lv, Kunying Chen, Yu Liu, Hanhong Lin, Shuhong Shen, Omar Abdel-Wahab, Shaoyan Hu, Zhaoqi Liu, Qianfei Wang
Cell Reports Medicine Available online: 20 January 2026
DOI:https://doi.org/10.1016/j.xcrm.2025.102542
Highlights
- Sequence-dependent splicing defects occur in 36% of pediatric AML
- U2AF2 downregulation drives aberrant splicing in pediatric AML
- Weak polypyrimidine tracts increase sensitivity to U2AF2 loss
- PRMT inhibition partially restores splicing and improves treatment response
Summary
Despite improvements in pediatric acute myeloid leukemia (AML) prognosis, about 30% of patients relapse after initial chemotherapy and have poor survival. However, the genetic basis of resistance remains unclear for most patients. To better understand the mechanistic basis and overcome treatment resistance, we analyze RNA sequencing (RNA-seq) data from 702 pediatric AML patients. This effort uncovers a sequence-dependent splicing dysregulation in 36% of children linked to worse prognosis and a lower rate of complete remission. Surprisingly, this change in RNA splicing matches that induced by SRSF2 mutations, which are common in adult AML. Instead, we identify U2AF2 dysregulation as the driver of aberrant splicing in pediatric AML. The pathologic splicing changes are characterized by “weak” polypyrimidine tracts and are susceptible to modest U2AF2 reduction. These outcomes can be improved by pharmacologic modulation of PRMT enzymes. Overall, these findings highlight the importance of modulating splicing defects to improve treatment response in pediatric AML.