2026-01-26 ブラウン大学
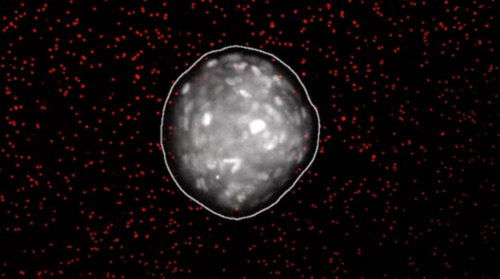
Imaging of the cells cultures showed that rotational behavior starts after around five hours. After 12 hours, cells began to invade out of the original sphere into the polymer matrix that contained them.
<関連情報>
- https://www.brown.edu/news/2026-01-26/cell-orbit-invasion
- https://www.nature.com/articles/s41567-025-03150-x
三次元多細胞球状体における軌道運動からマトリックス侵入への集団的遷移 Collective transitions from orbiting to matrix invasion in three-dimensional multicellular spheroids
Jiwon Kim,Hyuntae Jeong,Carles Falcó,Alex M. Hruska,W. Duncan Martinson,Alejandro Marzoratti,Mauricio Araiza,Haiqian Yang,Vera C. Fonseca,Stephen A. Adam,Christian Franck,José A. Carrillo,Ming Guo & Ian Y. Wong
Nature Physics Published:26 January 2026
DOI:https://doi.org/10.1038/s41567-025-03150-x
Abstract
Coordinated cell rotation along a curved matrix interface can sculpt epithelial tissues into spherical morphologies. Subsequently, radially oriented invasion of multicellular strands or branches can occur by local remodelling of the confining matrix. These symmetry-breaking transitions emerge from the dynamic reciprocity between cells and matrix but remain poorly understood. Here we show that epithelial cell spheroids collectively transition from circumferential orbiting to radial invasion via bidirectional interactions with the surrounding matrix curvature. Initially, spheroids exhibit an ellipsoidal shape but become rounded as orbiting occurs. In turn, orbiting along sharper curvature results in locally stronger contractile tractions, which gradually align collagen fibres in the radial direction. Thus, the initially elongated morphology primes the matrix towards subsequent invasion of two to four strands that are roughly aligned with its major axis. We then show that orbiting can be arrested and invasion can be reversed using osmotic pressure. We also investigate coordinated orbiting in mosaic spheroids, showing that a small fraction of cells with weakened cell–cell adhesions can impede collective orbiting but still invade into the matrix. This work elucidates how symmetry breaking in tissue morphogenesis is governed by the interplay of collective migration and the local curvature of the cell–matrix interface, with relevance for embryonic development and tumour progression.