反応性アストロサイトとニューロンの相互作用のPETイメージングにより、アルツハイマー病の病態に新たな知見が得られ、診断と治療のブレークスルーとなる可能性が示された PET imaging of reactive astrocyte-neuron interaction reveals new insights into Alzheimer’s disease pathology, offering a potential breakthrough in diagnosis and treatment
2023-04-17 韓国基礎科学研究院(IBS)
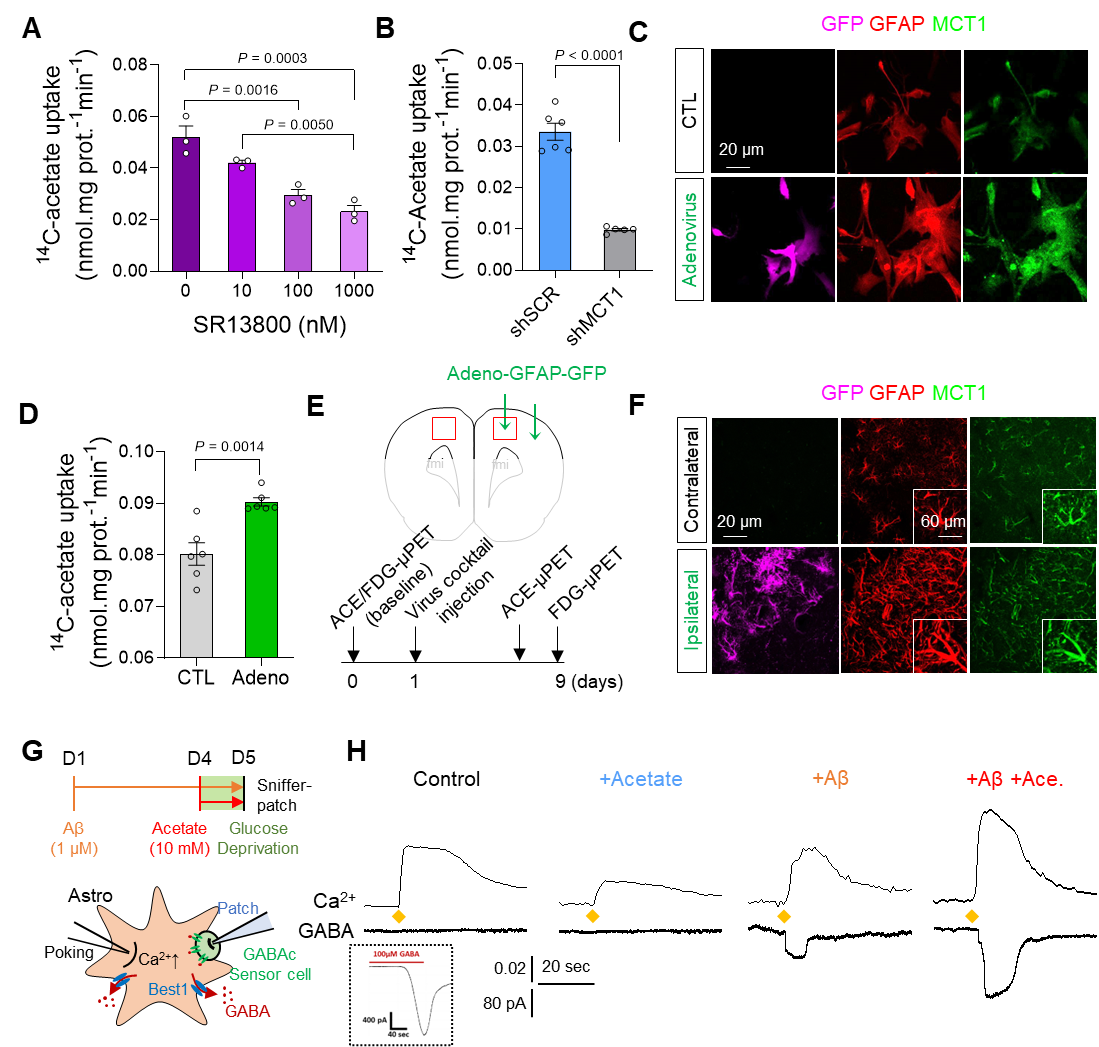 Figure 1. Augmentation of acetate uptake, mediated by MCT1, facilitates Aβ-induced GABA release in reactive astrocyte.
Figure 1. Augmentation of acetate uptake, mediated by MCT1, facilitates Aβ-induced GABA release in reactive astrocyte.
A. Blockade effect of MCT1 inhibitor on 14C-acetate uptake in primary cultured astrocytes.
B. Blockade effect of Mct1 gene-silencing on 14C-acetate uptake in primary cultured astrocytes.
C. Representative images displaying GFAP and MCT1 expressions in primary cultured astrocytes 48 hours after adenovirus treatment.
D. The adenovirus effect on 14C-acetate uptake.
E. Schematic diagram of in vivo micro-PET imaging of adenovirus model.
F. Representative images displaying GFAP and MCT1 expressions in adenovirus model.
G. Schematic diagram of sniffer patch to record GABA current.
H. Representative traces of Ca2+ signal (top) and GABA current (bottom).
PETイメージングにより、アルツハイマー病患者の神経代謝の変化を視覚化することができ、反応性星状グリア細胞との相互作用を直接観察することができる。これにより、アルツハイマー病の病態に新しい洞察を提供し、診断と治療において重要な突破口を開くことができる。
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do?nttId=22689&pageIndex=1&searchCnd=&searchWrd=
- https://academic.oup.com/brain/advance-article-abstract/doi/10.1093/brain/awad037/7117615
11C-acetateと18F-FDGを用いたアルツハイマー病における反応性アストロサイト・ニューロン相互作用の可視化 Visualizing reactive astrocyte-neuron interaction in Alzheimer’s disease using 11C-acetate and 18F-FDG
Min-Ho Nam, Hae Young Ko, Dongwoo Kim, Sangwon Lee, Yongmin Mason Park, Seung Jae Hyeon, Woojin Won, Jee-In Chung, Seon Yoo Kim, Han Hee Jo,Kyeong Taek Oh, Young-Eun Han, Gwan-Ho Lee, Yeon Ha Ju, Hyowon Lee, Hyunjin Kim, Jaejun Heo, Mridula Bhalla, Ki Jung Kim, Jea Kwon, Thor D Stein, Mingyu Kong, Hyunbeom Lee, Seung Eun Lee, Soo-Jin Oh, Joong-Hyun Chun, Mi-Ae Park, Ki Duk Park, Hoon Ryu, Mijin Yun, C Justin Lee
Brain Published:17 April 2023
DOI:https://doi.org/10.1093/brain/awad037
Abstract
Reactive astrogliosis is a hallmark of Alzheimer’s disease (AD). However, a clinically validated neuroimaging probe to visualize the reactive astrogliosis is yet to be discovered. Here, we show that PET imaging with 11C-acetate and 18F-fluorodeoxyglucose (18F-FDG) functionally visualizes the reactive astrocyte-mediated neuronal hypometabolism in the brains with neuroinflammation and AD.
To investigate the alterations of acetate and glucose metabolism in the diseased brains and their impact on the AD pathology, we adopted multifaceted approaches including microPET imaging, autoradiography, immunohistochemistry, metabolomics, and electrophysiology. Two AD rodent models, APP/PS1 and 5xFAD transgenic mice, one adenovirus-induced rat model of reactive astrogliosis, and post-mortem human brain tissues were used in this study. We further curated a proof-of-concept human study that included 11C-acetate and 18F-FDG PET imaging analyses along with neuropsychological assessments from 11 AD patients and 10 healthy control subjects.
We demonstrate that reactive astrocytes excessively absorb acetate through elevated monocarboxylate transporter-1 (MCT1) in rodent models of both reactive astrogliosis and AD. The elevated acetate uptake is associated with reactive astrogliosis and boosts the aberrant astrocytic GABA synthesis when amyloid-β is present. The excessive astrocytic GABA subsequently suppresses neuronal activity, which could lead to glucose uptake through decreased glucose transporter-3 in the diseased brains. We further demonstrate that 11C-acetate uptake was significantly increased in the entorhinal cortex, hippocampus and temporo-parietal neocortex of the AD patients compared to the healthy controls, while 18F-FDG uptake was significantly reduced in the same regions. Additionally, we discover a strong correlation between the patients’ cognitive function and the PET signals of both 11C-acetate and 18F-FDG.
We demonstrate the potential value of PET imaging with 11C-acetate and 18F-FDG by visualizing reactive astrogliosis and the associated neuronal glucose hypometablosim for AD patients. Our findings further suggest that the acetate-boosted reactive astrocyte-neuron interaction could contribute to the cognitive decline in AD.


