2023-04-18 チャルマース工科大学
そこで、スウェーデンのChalmers大学とFreiburg大学の研究者たちは、電気刺激を用いた傷の治癒促進法を開発し、糖尿病患者や高齢者など、傷が治癒しない人々にとって、ゲームチェンジャーになる可能性があるとしている。彼らは、傷口に電気刺激を与えることで、皮膚細胞を方向的に誘導し、傷の治癒を促進する方法を研究し、治癒に3倍の速度をもたらすことができると報告している。
<関連情報>
- https://news.cision.com/chalmers/r/how-electricity-can-heal-wounds-three-times-as-fast,c3750156
- https://pubs.rsc.org/en/content/articlelanding/2023/LC/D2LC01045C
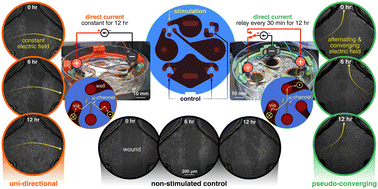
バイオエレクトロニクスマイクロ流体創傷治癒:傷ついた細胞集団の電流刺激を調べるためのプラットフォーム Bioelectronic microfluidic wound healing: a platform for investigating direct current stimulation of injured cell collectives
Sebastian Shaner, Anna Savelyeva, Anja Kvartuh, Nicole Jedrusik, Lukas Matter, José Leal and Maria Asplund
Lab on a Chip Published:18 Jan 2023
DOI:https://doi.org/10.1039/D2LC01045C
Abstract
Upon cutaneous injury, the human body naturally forms an electric field (EF) that acts as a guidance cue for relevant cellular and tissue repair and reorganization. However, the direct current (DC) flow imparted by this EF can be impacted by a variety of diseases. This work delves into the impact of DC stimulation on both healthy and diabetic in vitro wound healing models of human keratinocytes, the most prevalent cell type of the skin. The culmination of non-metal electrode materials and prudent microfluidic design allowed us to create a compact bioelectronic platform to study the effects of different sustained (12 hours galvanostatic DC) EF configurations on wound closure dynamics. Specifically, we compared if electrotactically closing a wound’s gap from one wound edge (i.e., uni-directional EF) is as effective as compared to alternatingly polarizing both the wound’s edges (i.e., pseudo-converging EF) as both of these spatial stimulation strategies are fundamental to the eventual translational electrode design and strategy. We found that uni-directional electric guidance cues were superior in group keratinocyte healing dynamics by enhancing the wound closure rate nearly three-fold for both healthy and diabetic-like keratinocyte collectives, compared to their non-stimulated respective controls. The motility-inhibited and diabetic-like keratinocytes regained wound closure rates with uni-directional electrical stimulation (increase from 1.0 to 2.8% h-1) comparable to their healthy non-stimulated keratinocyte counterparts (3.5% h-1). Our results bring hope that electrical stimulation delivered in a controlled manner can be a viable pathway to accelerate wound repair, and also by providing a baseline for other researchers trying to find an optimal electrode blueprint for in vivo DC stimulation.