2023-06-05 スイス連邦工科大学ローザンヌ校(EPFL)
◆研究者たちは、TDP43凝集物が脳内で病原性を示す前に、凝集物が処理されて「粘着性」の中心部が現れる必要があることを発見しました。この知見は、ALSや他の神経変性疾患の治療薬開発や早期診断法の開発に役立つ可能性があります。研究結果はNature Neuroscienceに発表されました。
<関連情報>
- https://actu.epfl.ch/news/how-a-highly-unstable-protein-may-lead-to-neurodeg/
- https://www.nature.com/articles/s41593-023-01341-4
TDP-43の凝集を促進するためには、線維化後のタンパク質分解が必要であることを発見 Seeding the aggregation of TDP-43 requires post-fibrillization proteolytic cleavage
Senthil T. Kumar,Sergey Nazarov,Sílvia Porta,Niran Maharjan,Urszula Cendrowska,Malek Kabani,Francesco Finamore,Yan Xu,Virginia M.-Y. Lee & Hilal A. Lashuel
Nature Neuroscience Published29 May 2023
DOI:https://doi.org/10.1038/s41593-023-01341-4
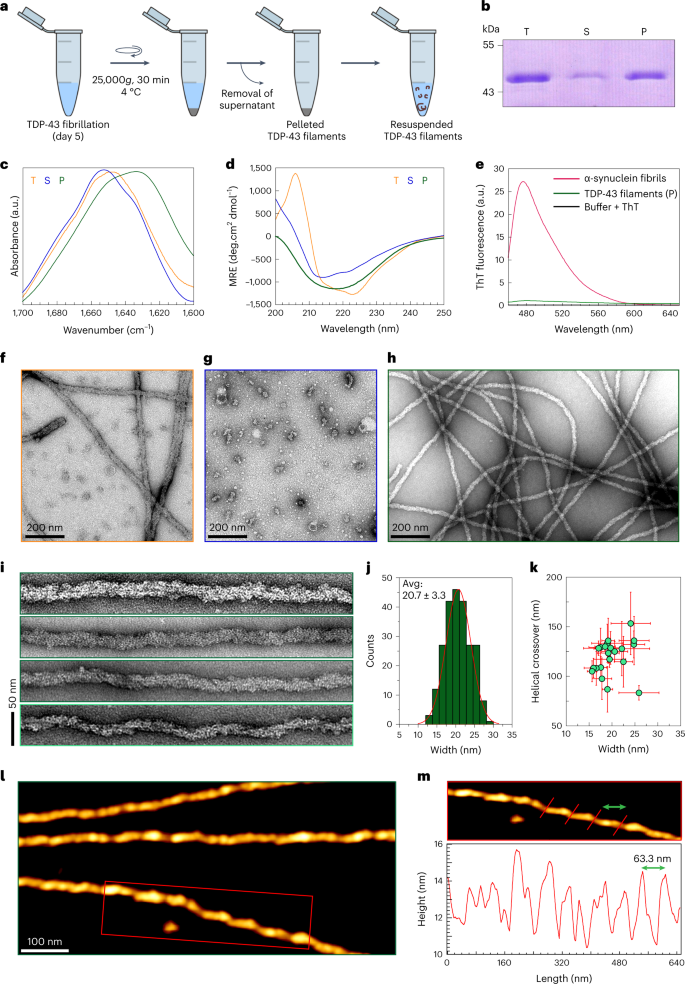
Abstract
Despite the strong evidence linking the transactive response DNA-binding protein 43 (TDP-43) aggregation to the pathogenesis of frontotemporal lobar degeneration with TDP-43, amyotrophic lateral sclerosis and several neurodegenerative diseases, our knowledge of the sequence and structural determinants of its aggregation and neurotoxicity remains incomplete. Herein, we present a new method for producing recombinant full-length TDP-43 filaments that exhibit sequence and morphological features similar to those of brain-derived TDP-43 filaments. We show that TDP-43 filaments contain a β-sheet-rich helical amyloid core that is fully buried by the flanking structured domains of the protein. We demonstrate that the proteolytic cleavage of TDP-43 filaments and exposure of this amyloid core are necessary for propagating TDP-43 pathology and enhancing the seeding of brain-derived TDP-43 aggregates. Only TDP-43 filaments with exposed amyloid core efficiently seeded the aggregation of endogenous TDP-43 in cells. These findings suggest that inhibiting the enzymes mediating cleavage of TDP-43 aggregates represents a viable disease-modifying strategy to slow the progression of amyotrophic lateral sclerosis and other TDP-43 proteinopathies.

