2023-08-02 カリフォルニア大学サンディエゴ校(UCSD)
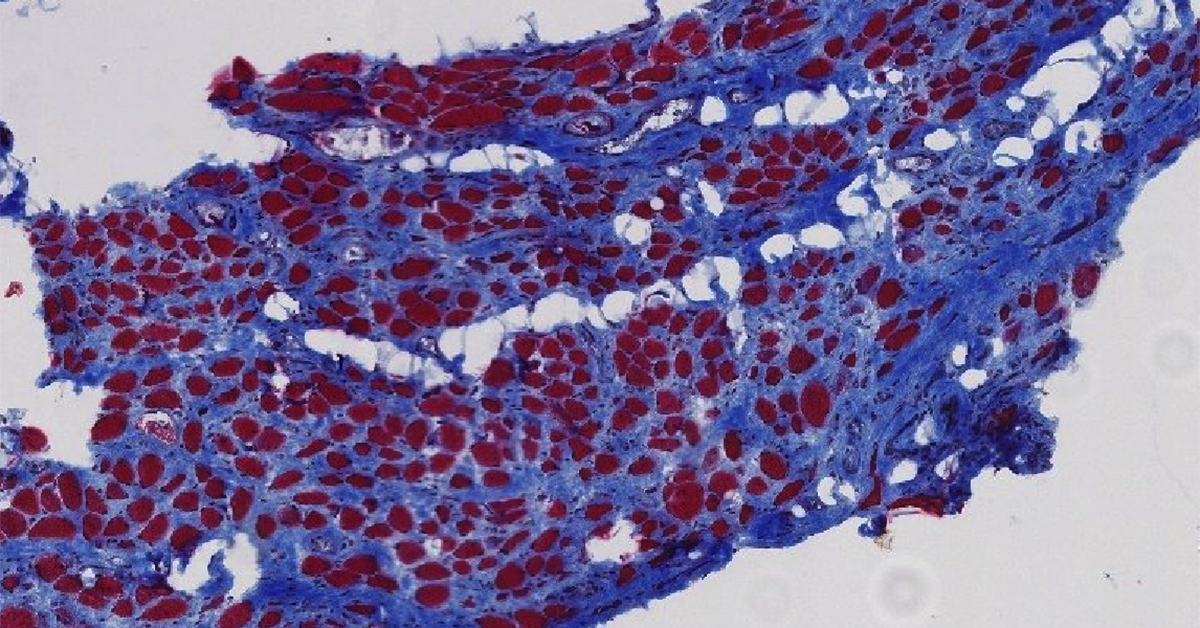 Biopsy cross sections of pelvic floor muscle in a woman with pelvic organ prolapse (POP) symptoms. The UC San Diego team found that pelvic floor muscles in women with symptomatic pelvic floor disorders show atrophy and fibrosis. This image is of stained biopsy tissue from a parous woman (who gave vaginal birth) with pelvic organ prolapse (POP). Blue stain shows intramuscular collagen content; while red stain shows muscle fibers. This new direct evidence of both atrophy and fibrosis in the skeletal muscles of the pelvic floor of women with symptoms of pelvic organ prolapse is an important step toward developing strategies to either prevent damage or aid recovery after damage occurs.
Biopsy cross sections of pelvic floor muscle in a woman with pelvic organ prolapse (POP) symptoms. The UC San Diego team found that pelvic floor muscles in women with symptomatic pelvic floor disorders show atrophy and fibrosis. This image is of stained biopsy tissue from a parous woman (who gave vaginal birth) with pelvic organ prolapse (POP). Blue stain shows intramuscular collagen content; while red stain shows muscle fibers. This new direct evidence of both atrophy and fibrosis in the skeletal muscles of the pelvic floor of women with symptoms of pelvic organ prolapse is an important step toward developing strategies to either prevent damage or aid recovery after damage occurs.
◆研究チームは、女性の骨盤底筋の検体とラットモデルを用いて、PFM機能障害に対する新たな治療法を探求しました。
◆実験結果は、膣分娩傷害による骨盤底筋の萎縮と線維症を明らかにし、ヒドロゲルを用いた治療がラットモデルで効果を示したことを示しています。これらの知見は、将来的な治療法や予防策の開発に向けて重要な一歩となるでしょう。
<関連情報>
- https://today.ucsd.edu/story/new-insights-on-pelvic-floor-damage-after-vaginal-birth-and-new-directions-for-treatment
- https://www.science.org/doi/10.1126/scitranslmed.abj3138
細胞外マトリックスハイドロゲルは、出生時の損傷後の骨盤骨格筋の病理学的変化を緩和する。 Proregenerative extracellular matrix hydrogel mitigates pathological alterations of pelvic skeletal muscles after birth injury
Pamela Duran,Francesca Boscolo Sesillo,Mark Cook,Lindsey Burnett,Shawn A. Menefee,Emmy Do,Saya French,Gisselle Zazueta-Damian,Monika Dzieciatkowska,Anthony J. Saviola,Manali M. Shah,Clyde Sanvictores,Kent G. Osborn,Kirk C. Hansen,Matthew Shtrahman,Karen L. Christman, and Marianna Alperin
Science Translational Medicine Published:2 Aug 2023
DOI:https://doi.org/10.1126/scitranslmed.abj3138
Editor’s summary
Pelvic floor muscle (PFM) dysfunction is a common disorder in women and is usually associated with vaginal childbirth, but the mechanisms underlying PFM dysfunction are not well understood. Here, Duran and colleagues studied human PFM samples and identified that the muscles atrophied and developed fibrosis in those with symptomatic pelvic organ prolapse. A rat model simulating birth injury to the pelvic muscles recapitulated human histopathology and demonstrated changes indicative of sustained inflammation and deposition of extracellular matrix (ECM) constituents. Treatment of these rats with an acellular injectable skeletal muscle ECM hydrogel at the time of or 4 weeks after simulated birth injury ameliorated these changes, suggesting that these hydrogels should be further investigated for the prevention of PFM dysfunction after birth injury. —Melissa Norton
Abstract
Pelvic floor disorders, including pelvic organ prolapse and urinary and fecal incontinence, affect millions of women globally and represent a major public health concern. Pelvic floor muscle (PFM) dysfunction has been identified as one of the leading risk factors for the development of these morbid conditions. Childbirth, specifically vaginal delivery, has been recognized as the most important potentially modifiable risk factor for PFM injury; however, the precise mechanisms of PFM dysfunction after parturition remain elusive. In this study, we demonstrated that PFMs exhibit atrophy and fibrosis in parous women with symptomatic pelvic organ prolapse. These pathological alterations were recapitulated in a preclinical rat model of simulated birth injury (SBI). The transcriptional signature of PFMs after injury demonstrated an impairment in muscle anabolism, persistent expression of genes that promote extracellular matrix (ECM) deposition, and a sustained inflammatory response. We also evaluated the administration of acellular injectable skeletal muscle ECM hydrogel for the prevention of these pathological alterations. Treatment of PFMs with the ECM hydrogel either at the time of birth injury or 4 weeks after injury mitigated PFM atrophy and fibrosis. By evaluating gene expression, we demonstrated that these changes are mainly driven by the hydrogel-induced enhancement of endogenous myogenesis, ECM remodeling, and modulation of the immune response. This work furthers our understanding of PFM birth injury and demonstrates proof of concept for future investigations of proregenerative biomaterial approaches for the treatment of injured pelvic soft tissues.

