2023-08-03 ペンシルベニア州立大学(PennState)
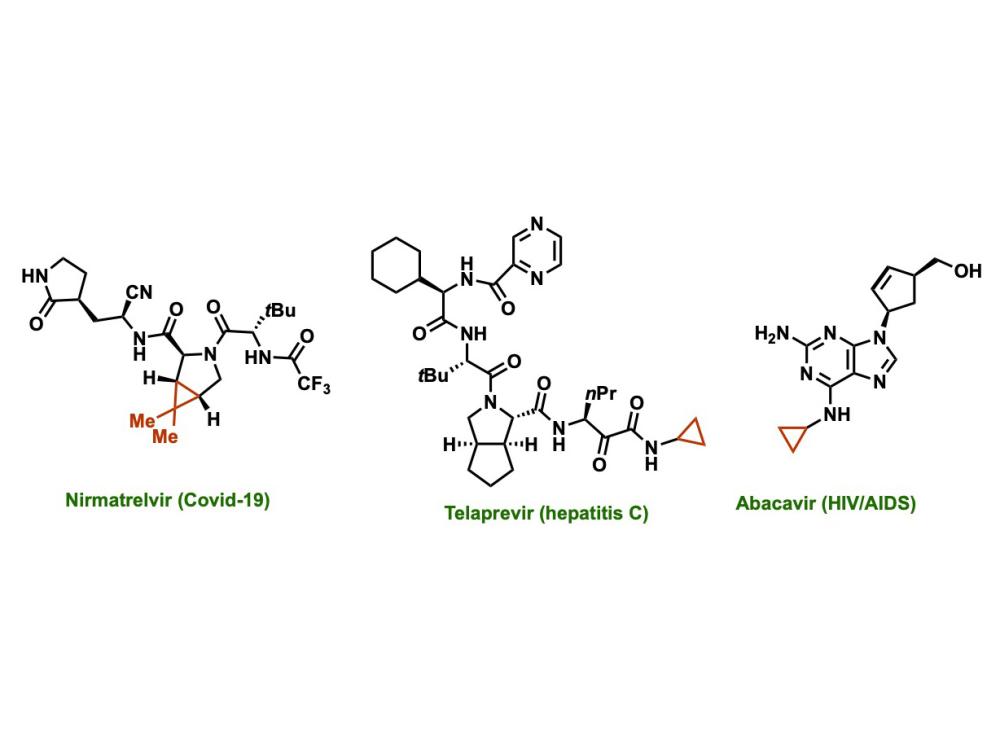 Cyclopropanes are ring of three connected carbon atoms that occur in many drugs currently approved by the FDA, including those used to treat COVID-19, asthma, hepatitis C, and HIV/AIDs. On a chemical diagram, they appear as a triangle (noted here in red), with a carbon atom at each point of the triangle. One of the carbon atoms is attached to the rest of the drug molecule and the other two are each attached to two hydrogen atoms. Credit: Giri Lab / Penn State. Creative Commons
Cyclopropanes are ring of three connected carbon atoms that occur in many drugs currently approved by the FDA, including those used to treat COVID-19, asthma, hepatitis C, and HIV/AIDs. On a chemical diagram, they appear as a triangle (noted here in red), with a carbon atom at each point of the triangle. One of the carbon atoms is attached to the rest of the drug molecule and the other two are each attached to two hydrogen atoms. Credit: Giri Lab / Penn State. Creative Commons
◆新しい方法は、薬の開発と創造におけるプロセスを変革する可能性を持つ。シクロプロパンは多くの承認済み薬剤に存在し、その性質を調整する役割を果たす。新しい方法は、従来の難解な過程を簡素化し、幅広い分子に適用可能なものであり、薬の研究に重要な影響をもたらす可能性がある。
<関連情報>
- https://www.psu.edu/news/eberly-college-science/story/new-simple-and-accessible-method-creates-potency-increasing-structure/
- https://www.science.org/doi/10.1126/science.adg3209
光増感O2が活性メチレン化合物による分子間アルケンシクロプロパン化を可能にする Photosensitized O2 enables intermolecular alkene cyclopropanation by active methylene compounds
Dhruba P. Poudel,Amrit Pokhrel,Raj Kumar Tak,Majji Shankar, and Ramesh Giri
Science Published:3 Aug 2023
DOI:https://doi.org/10.1126/science.adg3209
Editor’s summary
Strained three-carbon rings, or cyclopropanes, are important constituents of a wide variety of natural products, pharmaceuticals, and agrochemicals. However, most methods for their chemical synthesis unfortunately still rely on hazardous diazo reagents or sensitive doubly halogenated carbon centers. Poudel et al. now report a versatile cyclopropanation reaction using readily available, stable compounds with methylene centers bearing electron-withdrawing ketone, ester, or cyano groups. Activation of these precursors relies on photoreduction of oxygen by a sensitizer, with catalytic iodine to turn over the cycle. —Jake S. Yeston
Abstract
Cyclopropanes are key features in many preclinical, clinical, and commercial drugs, as well as natural products. The most prolific technique for their synthesis is the metal-catalyzed reaction of an alkene with a diazoalkane, a highly energetic reagent requiring stringent safety precautions. Discovery of alternative innocuous reagents remains an ongoing challenge. Herein, we report a simple photoredox-catalyzed intermolecular cyclopropanation of unactivated alkenes with active methylene compounds. The reaction proceeds in neutral solvent under air or dioxygen (O2) with a photoredox catalyst excited by blue light-emitting diode light and an iodine co-catalyst that is either added as molecular iodine or generated in situ from alkyl iodides. Mechanistic investigations indicate that photosensitized O2 plays a vital role in the generation of carbon-centered radicals for both the addition of active methylene compounds to alkenes and the ring closure.