2023-08-29 パデュー大学
◆研究では、衝撃後24時間以内に、神経細胞クラスター内のアクロレインレベルの上昇と異常なAB42の生成が350%増加することが示されました。この研究は、脳の健康に対する外傷の影響を明らかにするとともに、早期の対応の重要性を示唆しています。
<関連情報>
- https://www.purdue.edu/newsroom/releases/2023/Q3/a-mini-brain-traces-the-link-between-concussion-and-alzheimers-disease.html
- https://pubs.rsc.org/en/content/articlelanding/2023/lc/d3lc00248a
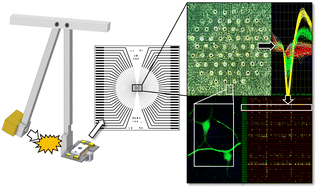
脳外傷(TBI)チップモデルを用いた神経細胞ネットワークにおけるβ-アミロイド蓄積と機能変化に対する初期脳震盪力とその結果生じるアクロレインサージの寄与 The contribution of initial concussive forces and resulting acrolein surge to β-amyloid accumulation and functional alterations in neuronal networks using a TBI-on-a-chip model
Edmond A. Rogers, Timothy Beauclair, Jhon Martinez, Shatha J. Mufti, David Kim, Siyuan Sun, Rachel L. Stingel, Alexandra M. Dieterly, Nikita Krishnan, Jennifer Crodian and Riyi Shi
Lab on a Chip Published:14 Jun 2023
DOI:https://doi.org/10.1039/D3LC00248A
Abstract
Trauma-induced Alzheimer’s disease (AD) is rapidly emerging as a major consequence of traumatic brain injuries (TBI), with devastating social and economic impacts. Unfortunately, few treatment options are currently available due to a limited understanding of the underlying mechanisms. A clinically-relevant, in vitro experimental model that emulates in vivo scenarios with high levels of spatial and temporal resolution is critical for demystifying the pathways of post-TBI AD. Using a unique, recently established “TBI-on-a-chip” system with murine cortical networks, we demonstrate the correlative elevation of oxidative stress (acrolein), inflammation (TNF-α), and Aβ42 aggregation, with concomitant reduction of neuronal network electrical activity post-concussive impact. These findings confirm that TBI-on-a-chip could provide a novel paradigm to supplement in vivo studies of trauma, while simultaneously validating the interaction of these alleged, key-pathological factors in post-TBI AD development. Specifically, we have shown that acrolein, acting as a diffusive factor of secondary injury, is both critical and sufficient in promoting inflammation (TNF-α) and Aβ42 aggregation, two known contributors of AD pathogenesis. Furthermore, using a cell-free preparation with TBI-on-a-chip, we have confirmed that both force and acrolein can independently and directly stimulate the aggregation of purified Aβ42, highlighting the key capabilities of primary and secondary injury mechanisms towards inducing Aβ42 aggregation, independently and synergistically. In addition to morphological and biochemical assessment, we also demonstrate parallel monitoring of neuronal network activity, further validating the chief pathological role of acrolein in not only inflicting biochemical abnormalities, but also functional deficits in neuronal networks. In conclusion, through this line of investigations, we have shown that by recapitulating clinically-relevant events, the TBI-on-a-chip device is capable of quantitatively characterizing parallel force-dependent increases in oxidative stress, inflammation, protein aggregation, and network activity, offering a unique platform for mechanistic investigations of post-TBI AD, and trauma-induced neuronal injury in general. It is expected that this model could provide crucial insights into pathological mechanisms which will be critical in developing novel, effective diagnostics and treatment strategies that significantly benefit TBI victims.