2023-08-30 インペリアル・カレッジ・ロンドン(ICL)
◆研究者はこのプロセスへの対応方法を研究中であり、抗生物質を服用中の患者には耐性菌の成長を制限するための抑制物質を与え、抗生物質の服用をやめた後には腸内の有益な細菌の混合物を提供する「微生物療法」を開発し、感染リスクを減少させ、患者の回復を助けることを目指しています。
<関連情報>
- https://www.imperial.ac.uk/news/247213/antibiotics-promote-growth-antibiotic-resistant-bacteria/
- https://www.nature.com/articles/s41467-023-40872-z
抗生物質がカルバペネム耐性腸内細菌科細菌の腸管内増殖を促進することが明らかになった Antibiotics promote intestinal growth of carbapenem-resistant Enterobacteriaceae by enriching nutrients and depleting microbial metabolites
Alexander Y. G. Yip,Olivia G. King,Oleksii Omelchenko,Sanjana Kurkimat,Victoria Horrocks,Phoebe Mostyn,Nathan Danckert,Rohma Ghani,Giovanni Satta,Elita Jauneikaite,Frances J. Davies,Thomas B. Clarke,Benjamin H. Mullish,Julian R. Marchesi & Julie A. K. McDonald
Nature Communications Published:22 August 2023
DOI:https://doi.org/10.1038/s41467-023-40872-z
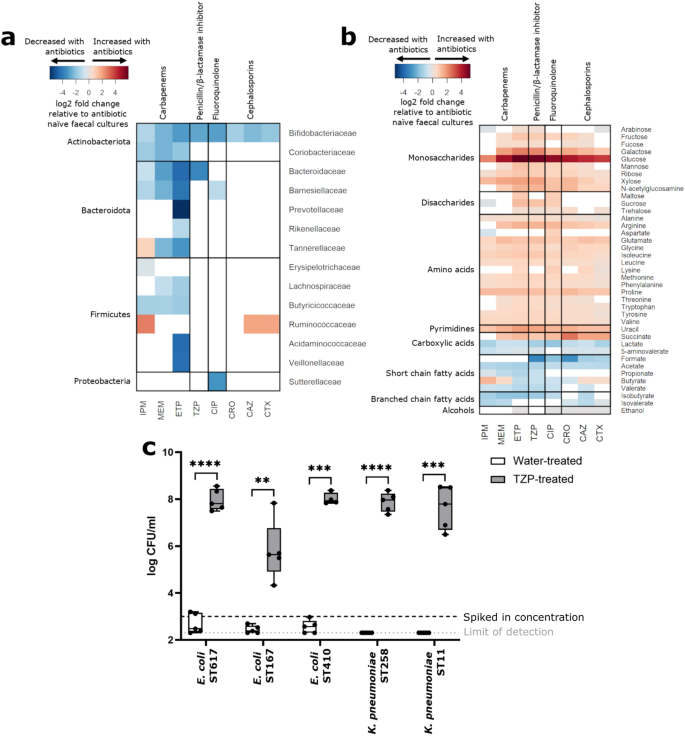
Abstract
The intestine is the primary colonisation site for carbapenem-resistant Enterobacteriaceae (CRE) and serves as a reservoir of CRE that cause invasive infections (e.g. bloodstream infections). Broad-spectrum antibiotics disrupt colonisation resistance mediated by the gut microbiota, promoting the expansion of CRE within the intestine. Here, we show that antibiotic-induced reduction of gut microbial populations leads to an enrichment of nutrients and depletion of inhibitory metabolites, which enhances CRE growth. Antibiotics decrease the abundance of gut commensals (including Bifidobacteriaceae and Bacteroidales) in ex vivo cultures of human faecal microbiota; this is accompanied by depletion of microbial metabolites and enrichment of nutrients. We measure the nutrient utilisation abilities, nutrient preferences, and metabolite inhibition susceptibilities of several CRE strains. We find that CRE can use the nutrients (enriched after antibiotic treatment) as carbon and nitrogen sources for growth. These nutrients also increase in faeces from antibiotic-treated mice and decrease following intestinal colonisation with carbapenem-resistant Escherichia coli. Furthermore, certain microbial metabolites (depleted upon antibiotic treatment) inhibit CRE growth. Our results show that killing gut commensals with antibiotics facilitates CRE colonisation by enriching nutrients and depleting inhibitory microbial metabolites.