2023-10-02 カロリンスカ研究所(KI)
◆通常難治性であるAMLに対して新たな希望を提供する可能性があり、5年生存率が低いAML患者にとって革新的な治療法となる可能性があります。免疫療法は、特定の変異を共有する患者に焦点を当てることで、AML治療における有望な選択肢として浮上しています。研究は多くの患者の細胞を用いて行われ、健康な細胞には影響を与えずにがんを除去することが示されました。
<関連情報>
- https://news.ki.se/immunotherapy-that-eliminates-mutated-cancer-cells-shows-promising-effect
- https://www.nature.com/articles/s43018-023-00642-8#citeas
FLT3の再発性ドライバー変異を標的とするAT細胞受容体は、生体内での原発性ヒト急性骨髄性白血病の排除を媒介する A T cell receptor targeting a recurrent driver mutation in FLT3 mediates elimination of primary human acute myeloid leukemia in vivo
Eirini Giannakopoulou,Madeleine Lehander,Stina Virding Culleton,Weiwen Yang,Yingqian Li,Terhi Karpanen,Tetsuichi Yoshizato,Even H. Rustad,Morten Milek Nielsen,Ravi Chand Bollineni,Trung T. Tran,Marina Delic-Sarac,Thea Johanne Gjerdingen,Karolos Douvlataniotis,Maarja Laos,Muhammad Ali,Amy Hillen,Stefania Mazzi,Desmond Wai Loon Chin,Adi Mehta,Jeppe Sejerø Holm,Amalie Kai Bentzen,Marie Bill,Marieke Griffioen,Tobias Gedde-Dahl,Sören Lehmann,Sten Eirik W. Jacobsen,Petter S. Woll & Johanna Olweus
Nature Cancer Published:02 October 2023
DOI:https://doi.org/10.1038/s43018-023-00642-8
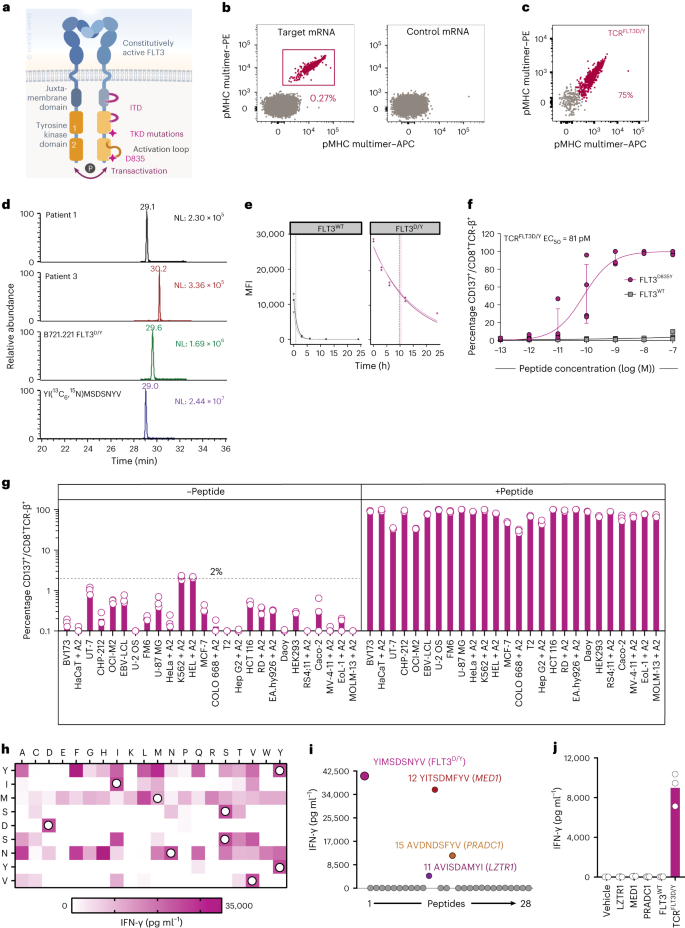
Abstract
Acute myeloid leukemia (AML), the most frequent leukemia in adults, is driven by recurrent somatically acquired genetic lesions in a restricted number of genes. Treatment with tyrosine kinase inhibitors has demonstrated that targeting of prevalent FMS-related receptor tyrosine kinase 3 (FLT3) gain-of-function mutations can provide significant survival benefits for patients, although the efficacy of FLT3 inhibitors in eliminating FLT3-mutated clones is variable. We identified a T cell receptor (TCR) reactive to the recurrent D835Y driver mutation in the FLT3 tyrosine kinase domain (TCRFLT3D/Y). TCRFLT3D/Y-redirected T cells selectively eliminated primary human AML cells harboring the FLT3D835Y mutation in vitro and in vivo. TCRFLT3D/Y cells rejected both CD34+ and CD34– AML in mice engrafted with primary leukemia from patients, reaching minimal residual disease-negative levels, and eliminated primary CD34+ AML leukemia-propagating cells in vivo. Thus, T cells targeting a single shared mutation can provide efficient immunotherapy toward selective elimination of clonally involved primary AML cells in vivo.