2023-10-24 ニューサウスウェールズ大学(UNSW)
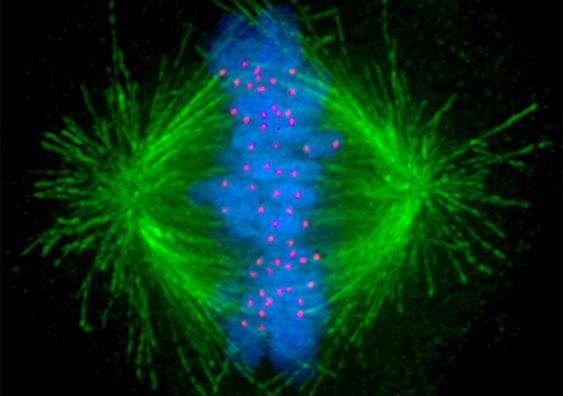
Tiny tube-like structures inside the cell called microtubules (in green) that help separate chromosomes evenly during cell division can overcome low exposures to chemotherapy. Image: Wikimedia Commons.
◆研究によれば、がん細胞は、化学療法の影響に耐え、細胞分裂に責任がある重要な細胞構造を安定させるための力を発生する救助メカニズムを活性化しています。これにより、化学療法の影響を回避し、がん細胞の増殖が可能になります。研究チームは、このメカニズムを利用してがん治療を改善し、特定のメカニズムを標的とする薬剤を開発するためにこの発見を活用しようとしており、既にこのメカニズムを特定する薬剤を開発する会社を設立しています。この発見により、がん細胞の治療への新たなアプローチが可能になると期待されています。
<関連情報>
- https://newsroom.unsw.edu.au/news/health/we-could-see-it-happening-our-eyes-research-shows-how-cancer-cells-resist-chemotherapy
- https://www.sciencedirect.com/science/article/abs/pii/S096098222301268X
細胞外マトリックスに含まれるトリプトファンジッパーペプチドにストレス緩和作用があることが示唆される Cortical tension drug screen links mitotic spindle integrity to Rho pathway
Dejiang Wang, Yao Wang, Xiangjun Di, Fan Wang, Amanda Wanninayaka, Michael Carnell, Edna C. Hardeman, Dayong Jin, Peter W. Gunning
Current Biology Published: October 23, 2023
DOI:https://doi.org/10.1016/j.cub.2023.09.022
Summary
Mechanical force generation plays an essential role in many cellular functions, including mitosis. Actomyosin contractile forces mediate changes in cell shape in mitosis and are implicated in mitotic spindle integrity via cortical tension. An unbiased screen of 150 small molecules that impact actin organization and 32 anti-mitotic drugs identified two molecular targets, Rho kinase (ROCK) and tropomyosin 3.1/2 (Tpm3.1/2), whose inhibition has the greatest impact on mitotic cortical tension. The converse was found for compounds that depolymerize microtubules. Tpm3.1/2 forms a co-polymer with mitotic cortical actin filaments, and its inhibition prevents rescue of multipolar spindles induced by anti-microtubule chemotherapeutics. We examined the role of mitotic cortical tension in this rescue mechanism. Inhibition of ROCK and Tpm3.1/2 and knockdown (KD) of cortical nonmuscle myosin 2A (NM2A), all of which reduce cortical tension, inhibited rescue of multipolar mitotic spindles, further implicating cortical tension in the rescue mechanism. GEF-H1 released from microtubules by depolymerization increased cortical tension through the RhoA pathway, and its KD also inhibited rescue of multipolar mitotic spindles. We conclude that microtubule depolymerization by anti-cancer drugs induces cortical-tension-based rescue to ensure integrity of the mitotic bipolar spindle mediated via the RhoA pathway. Central to this mechanism is the dependence of NM2A on Tpm3.1/2 to produce the functional engagement of actin filaments responsible for cortical tension.