2023-11-01 カロリンスカ研究所(KI)
◆研究では、アルツハイマー病の病理学的変化をヒトと似たように進行させるマウスを使用し、若いマウスでは代謝の増加が、細胞のリサイクルシステムによる細胞の変化に続いた。
◆アルツハイマー病の脳の代謝は通常減少し、これがシナプスの劣化に寄与する。この疾患は症状の発現の20年前から進行するため、早期の検出が重要であり、代謝変化はそれを診断する際の重要な要因となりえる。
◆研究は、アルツハイマー病の特徴的な不溶性プラークが脳に蓄積する前にも代謝の変化が現れることを示している。今後の研究では、ミトコンドリアとオートファジーのアルツハイマー病への影響を詳細に調査し、新しい分子が疾患の進行を遅延させる可能性を検証する予定です。
<関連情報>
- https://news.ki.se/high-metabolism-is-an-early-sign-of-alzheimers-disease
- https://www.nature.com/articles/s41380-023-02289-4
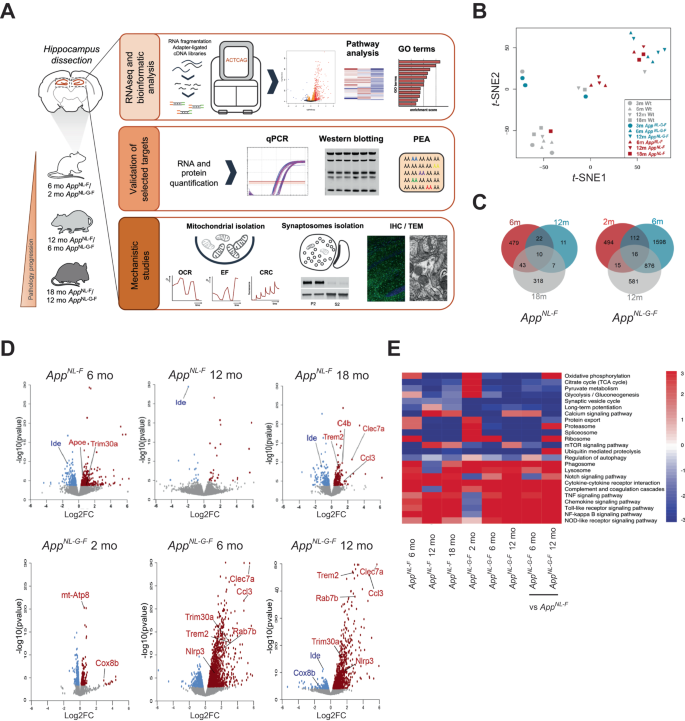
ミトコンドリア代謝亢進は、Appノックインアルツハイマー病モデルマウスにおけるオートファジー障害とシナプス崩壊に先行する Mitochondrial hypermetabolism precedes impaired autophagy and synaptic disorganization in App knock-in Alzheimer mouse models
Luana Naia,Makoto Shimozawa,Erika Bereczki,Xidan Li,Jianping Liu,Richeng Jiang,Romain Giraud,Nuno Santos Leal,Catarina Moreira Pinho,Erik Berger,Victoria Lim Falk,Giacomo Dentoni,Maria Ankarcrona & Per Nilsson
Molecular Psychiatry Published:01 November 2023
DOI:https://doi.org/10.1038/s41380-023-02289-4
Abstract
Accumulation of amyloid β-peptide (Aβ) is a driver of Alzheimer’s disease (AD). Amyloid precursor protein (App) knock-in mouse models recapitulate AD-associated Aβ pathology, allowing elucidation of downstream effects of Aβ accumulation and their temporal appearance upon disease progression. Here we have investigated the sequential onset of AD-like pathologies in AppNL-F and AppNL-G-F knock-in mice by time-course transcriptome analysis of hippocampus, a region severely affected in AD. Strikingly, energy metabolism emerged as one of the most significantly altered pathways already at an early stage of pathology. Functional experiments in isolated mitochondria from hippocampus of both AppNL-F and AppNL-G-F mice confirmed an upregulation of oxidative phosphorylation driven by the activity of mitochondrial complexes I, IV and V, associated with higher susceptibility to oxidative damage and Ca2+-overload. Upon increasing pathologies, the brain shifts to a state of hypometabolism with reduced abundancy of mitochondria in presynaptic terminals. These late-stage mice also displayed enlarged presynaptic areas associated with abnormal accumulation of synaptic vesicles and autophagosomes, the latter ultimately leading to local autophagy impairment in the synapses. In summary, we report that Aβ-induced pathways in App knock-in mouse models recapitulate key pathologies observed in AD brain, and our data herein adds a comprehensive understanding of the pathologies including dysregulated metabolism and synapses and their timewise appearance to find new therapeutic approaches for AD.