2023-11-10 インペリアル・カレッジ・ロンドン(ICL)
◆Francis Crick Institute率いる研究チームは、Mycと呼ばれる主要ながん促進遺伝子に焦点を当て、ビタミンB5がヒトおよびマウスの腫瘍において高いMyc発現領域と関連していることを示しました。さらに、ビタミンB5の制限が腫瘍の成長を遅らせる効果があり、これはビタミンB5が細胞の代謝において果たす鍵となる役割に起因していると考えられています。
◆この研究から、腫瘍の代謝が治療の対象となりうる「脆弱性」である可能性が浮かび上がり、将来的な治療法の開発に繋がる可能性があります。
<関連情報>
- https://www.imperial.ac.uk/news/249434/bendy-x-rays-dmt-infusions-news-from/
- https://www.nature.com/articles/s42255-023-00915-7
ビタミンB5は乳癌におけるMYC発癌代謝と腫瘍進行をサポートする Vitamin B5 supports MYC oncogenic metabolism and tumor progression in breast cancer
Peter Kreuzaler,Paolo Inglese,Avinash Ghanate,Ersa Gjelaj,Vincen Wu,Yulia Panina,Andres Mendez-Lucas,Catherine MacLachlan,Neill Patani,Catherine B. Hubert,Helen Huang,Gina Greenidge,Oscar M. Rueda,Adam J. Taylor,Evdoxia Karali,Emine Kazanc,Amy Spicer,Alex Dexter,Wei Lin,Daria Thompson,Mariana Silva Dos Santos,Enrica Calvani,Nathalie Legrave,James K. Ellis,Wendy Greenwood,Mary Green,Emma Nye,Emma Still,CRUK Rosetta Grand Challenge Consortium,Simon Barry,Richard J. A. Goodwin,Alejandra Bruna,Carlos Caldas,James MacRae,Luiz Pedro Sório de Carvalho,George Poulogiannis,Greg McMahon,Zoltan Takats,Josephine Bunch & Mariia Yuneva
Nature Metabolism Published:09 November 2023
DOI:https://doi.org/10.1038/s42255-023-00915-7
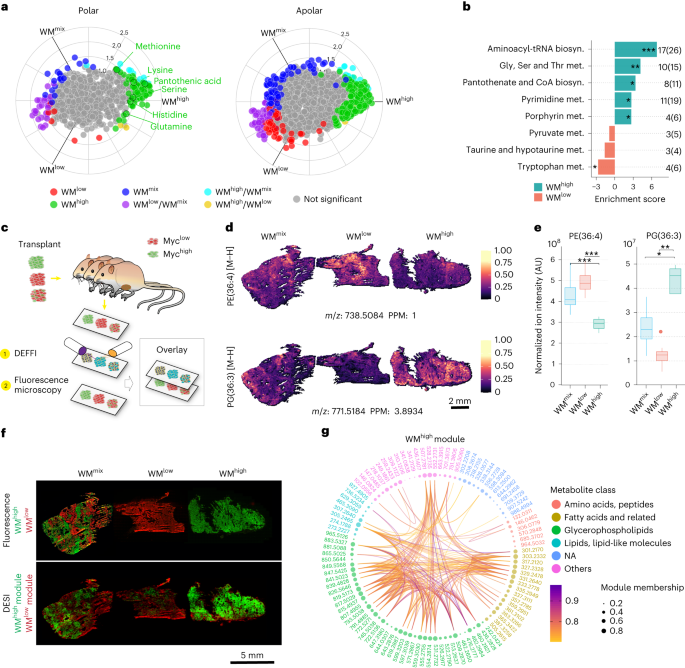
Abstract
Tumors are intrinsically heterogeneous and it is well established that this directs their evolution, hinders their classification and frustrates therapy1,2,3. Consequently, spatially resolved omics-level analyses are gaining traction4,5,6,7,8,9. Despite considerable therapeutic interest, tumor metabolism has been lagging behind this development and there is a paucity of data regarding its spatial organization. To address this shortcoming, we set out to study the local metabolic effects of the oncogene c-MYC, a pleiotropic transcription factor that accumulates with tumor progression and influences metabolism10,11. Through correlative mass spectrometry imaging, we show that pantothenic acid (vitamin B5) associates with MYC-high areas within both human and murine mammary tumors, where its conversion to coenzyme A fuels Krebs cycle activity. Mechanistically, we show that this is accomplished by MYC-mediated upregulation of its multivitamin transporter SLC5A6. Notably, we show that SLC5A6 over-expression alone can induce increased cell growth and a shift toward biosynthesis, whereas conversely, dietary restriction of pantothenic acid leads to a reversal of many MYC-mediated metabolic changes and results in hampered tumor growth. Our work thus establishes the availability of vitamins and cofactors as a potential bottleneck in tumor progression, which can be exploited therapeutically. Overall, we show that a spatial understanding of local metabolism facilitates the identification of clinically relevant, tractable metabolic targets.


