2023-11-24 スイス連邦工科大学ローザンヌ校(EPFL)
◆最新の研究では、新たに開発されたエンジニアリングされたDCsが抗原の積み込みなしで効果的な抗腫瘍免疫応答を引き起こすことが示され、CD8 T細胞を含む免疫系の広範な要素と連携してがん治療に有望な成果を上げたと報告されました。
<関連情報>
- https://actu.epfl.ch/news/treating-tumors-with-engineered-dendritic-cells/
- https://www.nature.com/articles/s43018-023-00668-y
抗原診断的がん免疫療法のためのサイトカインで武装した樹状細胞前駆細胞 Cytokine-armed dendritic cell progenitors for antigen-agnostic cancer immunotherapy
Ali Ghasemi,Amaia Martinez-Usatorre,Luqing Li,Mehdi Hicham,Alan Guichard,Rachel Marcone,Nadine Fournier,Bruno Torchia,Darel Martinez Bedoya,Suzel Davanture,Mirian Fernández-Vaquero,Chaofan Fan,Jakob Janzen,Yahya Mohammadzadeh,Raphael Genolet,Nahal Mansouri,Mathias Wenes,Denis Migliorini,Mathias Heikenwalder & Michele De Palma
Nature Cancer Published:23 November 2023
DOI:https://doi.org/10.1038/s43018-023-00668-y
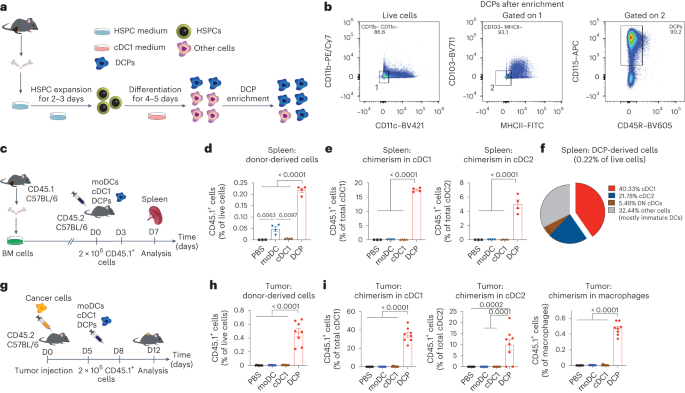
Abstract
Dendritic cells (DCs) are antigen-presenting myeloid cells that regulate T cell activation, trafficking and function. Monocyte-derived DCs pulsed with tumor antigens have been tested extensively for therapeutic vaccination in cancer, with mixed clinical results. Here, we present a cell-therapy platform based on mouse or human DC progenitors (DCPs) engineered to produce two immunostimulatory cytokines, IL-12 and FLT3L. Cytokine-armed DCPs differentiated into conventional type-I DCs (cDC1) and suppressed tumor growth, including melanoma and autochthonous liver models, without the need for antigen loading or myeloablative host conditioning. Tumor response involved synergy between IL-12 and FLT3L and was associated with natural killer and T cell infiltration and activation, M1-like macrophage programming and ischemic tumor necrosis. Antitumor immunity was dependent on endogenous cDC1 expansion and interferon-γ signaling but did not require CD8+ T cell cytotoxicity. Cytokine-armed DCPs synergized effectively with anti-GD2 chimeric-antigen receptor (CAR) T cells in eradicating intracranial gliomas in mice, illustrating their potential in combination therapies.