2023-12-04 マックス・プランク研究所
◆バルセロナ大学の研究チームは、転写因子が生物分子凝集物を形成する性質を利用し、前立腺がんのアンドロゲン受容体活性を抑制する方法を発見しました。この手法は、他の転写因子にも応用可能で、新しい治療法の可能性が広がります。バイオテクノロジー企業Nuage Therapeuticsは、これらの研究を基にし、難治性疾患への新しい治療法を提供するために設立されました。
<関連情報>
- https://www.mpg.de/21203211/1204-moge-hard-to-drug-protein-droplets-reveal-new-ways-to-inhibit-transcription-factors-in-an-aggressive-form-of-prostate-cancer-151795-x
- https://www.nature.com/articles/s41594-023-01159-5
転写因子活性化ドメイン阻害剤の合理的最適化 Rational optimization of a transcription factor activation domain inhibitor
Shaon Basu,Paula Martínez-Cristóbal,Marta Frigolé-Vivas,Mireia Pesarrodona,Michael Lewis,Elzbieta Szulc,C. Adriana Bañuelos,Carolina Sánchez-Zarzalejo,Stasė Bielskutė,Jiaqi Zhu,Karina Pombo-García,Carla Garcia-Cabau,Levente Zodi,Hannes Dockx,Jordann Smak,Harpreet Kaur,Cristina Batlle,Borja Mateos,Mateusz Biesaga,Albert Escobedo,Lídia Bardia,Xavier Verdaguer,Alessandro Ruffoni,Nasrin R. Mawji,Jun Wang,Jon K. Obst,Teresa Tam,Isabelle Brun-Heath,Salvador Ventura,David Meierhofer,Jesús García,Paul Robustelli,Travis H. Stracker,Marianne D. Sadar,Antoni Riera,Denes Hnisz & Xavier Salvatella
Nature Structural & Molecular Biology Published:04 December 2023
DOI:https://doi.org/10.1038/s41594-023-01159-5
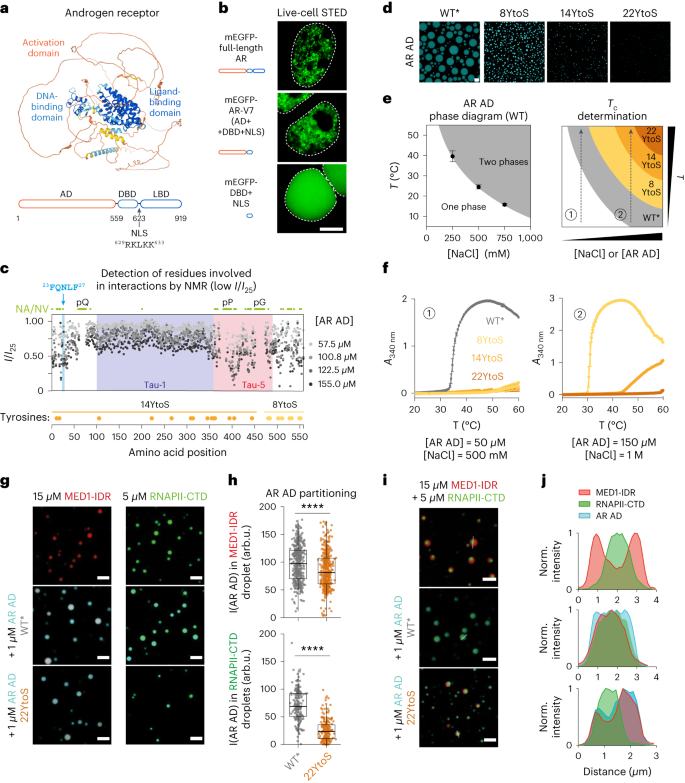
Abstract
Transcription factors are among the most attractive therapeutic targets but are considered largely ‘undruggable’ in part due to the intrinsically disordered nature of their activation domains. Here we show that the aromatic character of the activation domain of the androgen receptor, a therapeutic target for castration-resistant prostate cancer, is key for its activity as transcription factor, allowing it to translocate to the nucleus and partition into transcriptional condensates upon activation by androgens. On the basis of our understanding of the interactions stabilizing such condensates and of the structure that the domain adopts upon condensation, we optimized the structure of a small-molecule inhibitor previously identified by phenotypic screening. The optimized compounds had more affinity for their target, inhibited androgen-receptor-dependent transcriptional programs, and had an antitumorigenic effect in models of castration-resistant prostate cancer in cells and in vivo. These results suggest that it is possible to rationally optimize, and potentially even to design, small molecules that target the activation domains of oncogenic transcription factors.