2023-12-01 テキサス大学オースチン校(UT Austin)
◆このデジタルツインは個々の患者に合わせて初期化され、ベイジアンモデルによる補正とMRIデータの連続的な統合により、患者の治療反応を予測します。ベイジアンフレームワークの利用により、患者データと治療応答の不確実性を考慮し、がん治療における重要な意思決定をサポートしています。
◆この手法により、患者に合わせた治療計画が可能となり、放射線療法の適正な調整が行え、患者への副作用が減少します。初期の結果では、患者固有のデジタルツインを用いた治療が、標準治療よりも腫瘍の進行を約6日間遅らせる効果があることが示されています。
<関連情報>
- https://oden.utexas.edu/news-and-events/news/digital-twins-brain-tumors-computational-scientists-cancer-care/
- https://www.frontiersin.org/articles/10.3389/frai.2023.1222612/full
高悪性度神経膠腫における不確実性のもとでの患者特異的放射線治療レジメンの最適化のための予測的デジタルツイン
Predictive digital twin for optimizing patient-specific radiotherapy regimens under uncertainty in high-grade gliomas
Anirban Chaudhuri, Graham Pash, David A. Hormuth II, Guillermo Lorenzo, Michael Kapteyn, Chengyue Wu, Ernesto A. B. F. Lima, Thomas E. Yankeelov, Karen Willcox
Frontiers in Artificial Intelligence Published:11 October 2023
DOI:https://doi.org/10.3389/frai.2023.1222612
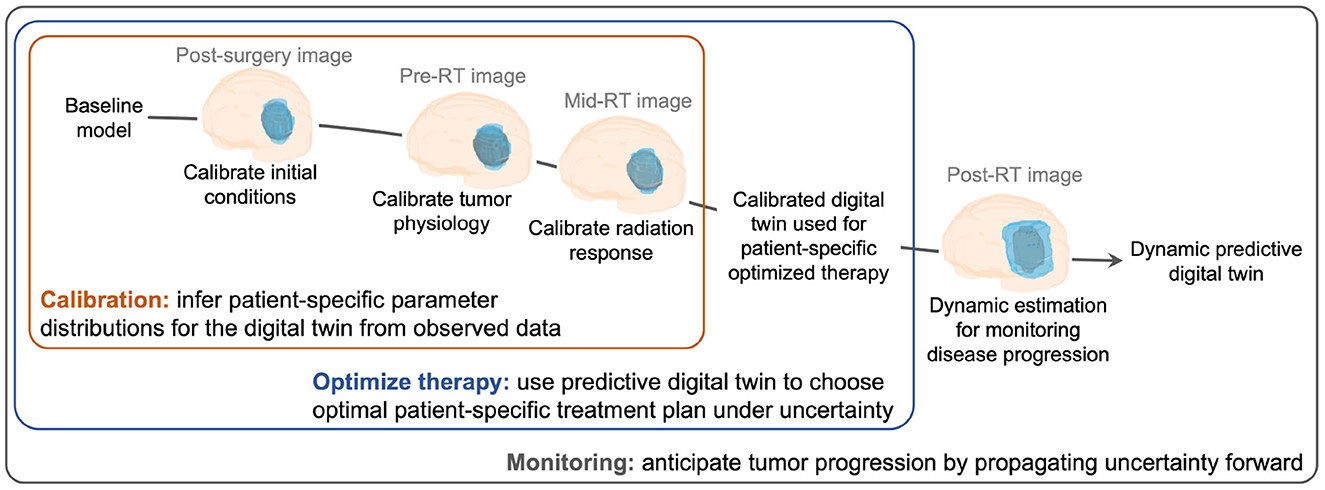
We develop a methodology to create data-driven predictive digital twins for optimal risk-aware clinical decision-making. We illustrate the methodology as an enabler for an anticipatory personalized treatment that accounts for uncertainties in the underlying tumor biology in high-grade gliomas, where heterogeneity in the response to standard-of-care (SOC) radiotherapy contributes to sub-optimal patient outcomes. The digital twin is initialized through prior distributions derived from population-level clinical data in the literature for a mechanistic model’s parameters. Then the digital twin is personalized using Bayesian model calibration for assimilating patient-specific magnetic resonance imaging data. The calibrated digital twin is used to propose optimal radiotherapy treatment regimens by solving a multi-objective risk-based optimization under uncertainty problem. The solution leads to a suite of patient-specific optimal radiotherapy treatment regimens exhibiting varying levels of trade-off between the two competing clinical objectives: (i) maximizing tumor control (characterized by minimizing the risk of tumor volume growth) and (ii) minimizing the toxicity from radiotherapy. The proposed digital twin framework is illustrated by generating an in silico cohort of 100 patients with high-grade glioma growth and response properties typically observed in the literature. For the same total radiation dose as the SOC, the personalized treatment regimens lead to median increase in tumor time to progression of around six days. Alternatively, for the same level of tumor control as the SOC, the digital twin provides optimal treatment options that lead to a median reduction in radiation dose by 16.7% (10 Gy) compared to SOC total dose of 60 Gy. The range of optimal solutions also provide options with increased doses for patients with aggressive cancer, where SOC does not lead to sufficient tumor control.