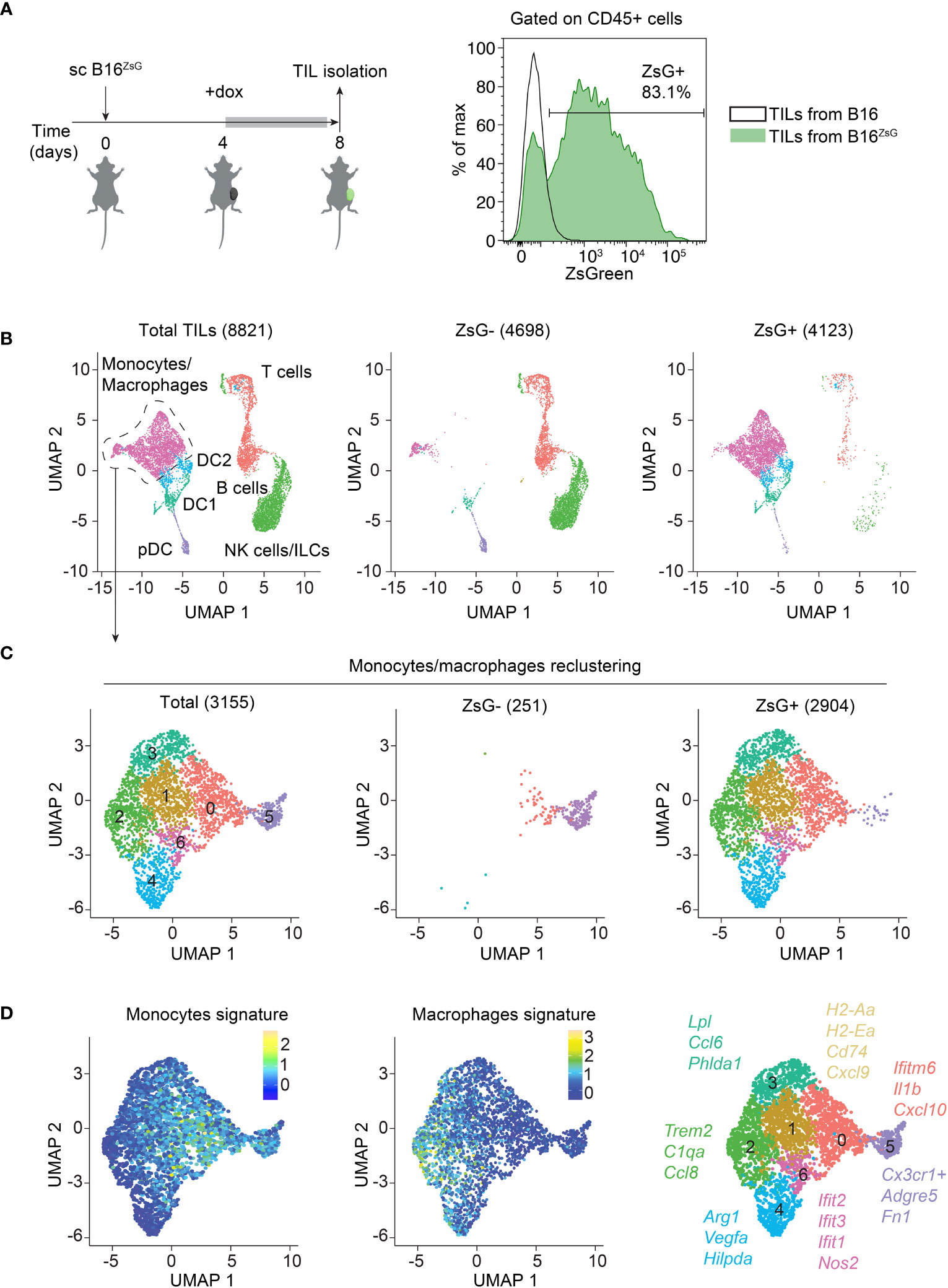
2024-01-08 マサチューセッツ大学アマースト校
◆電子タバコはタバコを燃やさず、ニコチンとフレーバリングを加熱して蒸気を吸引します。これにより、喫煙に伴う有害な化学物質に晒されることなく、禁煙を支援できます。
◆研究は、電子タバコを使用すると、100人中8〜10人が成功して喫煙をやめる可能性があると示しています。これは従来のNRTや支援なしの場合よりも高い数字です。
<関連情報>
- https://www.umass.edu/news/article/e-cigarettes-help-more-tobacco-smokers-quit-traditional-nicotine-replacement
- https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD010216.pub8/full
禁煙のための電子タバコ Electronic cigarettes for smoking cessation
Nicola Lindson,Ailsa R Butler,Hayden McRobbie,Chris Bullen,Peter Hajek,Rachna Begh,Annika Theodoulou,Caitlin Notley,Nancy A Rigotti,Tari Turner,Jonathan Livingstone-Banks,Tom Morris,Jamie Hartmann-Boyce
Cochrane Database of Systematic Reviews Published: 08 January 2024
DOI:https://doi.org/10.1002/14651858.CD010216.pub8
Abstract
Background
Electronic cigarettes (ECs) are handheld electronic vaping devices which produce an aerosol by heating an e‐liquid. People who smoke, healthcare providers and regulators want to know if ECs can help people quit smoking, and if they are safe to use for this purpose. This is a review update conducted as part of a living systematic review.
Objectives
To examine the safety, tolerability and effectiveness of using electronic cigarettes (ECs) to help people who smoke tobacco achieve long‐term smoking abstinence, in comparison to non‐nicotine EC, other smoking cessation treatments and no treatment.
Search methods
We searched the Cochrane Tobacco Addiction Group’s Specialized Register to 1 February 2023, and Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and PsycINFO to 1 July 2023, and reference‐checked and contacted study authors.
Selection criteria
We included trials in which people who smoke were randomized to an EC or control condition. We also included uncontrolled intervention studies in which all participants received an EC intervention as these studies have the potential to provide further information on harms and longer‐term use. Studies had to report an eligible outcome.
Data collection and analysis
We followed standard Cochrane methods for screening and data extraction. Critical outcomes were abstinence from smoking after at least six months, adverse events (AEs), and serious adverse events (SAEs). We used a fixed‐effect Mantel‐Haenszel model to calculate risk ratios (RRs) with a 95% confidence interval (CI) for dichotomous outcomes. For continuous outcomes, we calculated mean differences. Where appropriate, we pooled data in pairwise and network meta‐analyses (NMA).
Main results
We included 88 completed studies (10 new to this update), representing 27,235 participants, of which 47 were randomized controlled trials (RCTs). Of the included studies, we rated ten (all but one contributing to our main comparisons) at low risk of bias overall, 58 at high risk overall (including all non‐randomized studies), and the remainder at unclear risk.
There is high certainty that nicotine EC increases quit rates compared to nicotine replacement therapy (NRT) (RR 1.59, 95% CI 1.29 to 1.93; I2 = 0%; 7 studies, 2544 participants). In absolute terms, this might translate to an additional four quitters per 100 (95% CI 2 to 6 more). There is moderate‐certainty evidence (limited by imprecision) that the rate of occurrence of AEs is similar between groups (RR 1.03, 95% CI 0.91 to 1.17; I2 = 0%; 5 studies, 2052 participants). SAEs were rare, and there is insufficient evidence to determine whether rates differ between groups due to very serious imprecision (RR 1.20, 95% CI 0.90 to 1.60; I2 = 32%; 6 studies, 2761 participants; low‐certainty evidence).
There is moderate‐certainty evidence, limited by imprecision, that nicotine EC increases quit rates compared to non‐nicotine EC (RR 1.46, 95% CI 1.09 to 1.96; I2 = 4%; 6 studies, 1613 participants). In absolute terms, this might lead to an additional three quitters per 100 (95% CI 1 to 7 more). There is moderate‐certainty evidence of no difference in the rate of AEs between these groups (RR 1.01, 95% CI 0.91 to 1.11; I2 = 0%; 5 studies, 1840 participants). There is insufficient evidence to determine whether rates of SAEs differ between groups, due to very serious imprecision (RR 1.00, 95% CI 0.56 to 1.79; I2 = 0%; 9 studies, 1412 participants; low‐certainty evidence).
Due to issues with risk of bias, there is low‐certainty evidence that, compared to behavioural support only/no support, quit rates may be higher for participants randomized to nicotine EC (RR 1.88, 95% CI 1.56 to 2.25; I2 = 0%; 9 studies, 5024 participants). In absolute terms, this represents an additional four quitters per 100 (95% CI 2 to 5 more). There was some evidence that (non‐serious) AEs may be more common in people randomized to nicotine EC (RR 1.22, 95% CI 1.12 to 1.32; I2 = 41%, low‐certainty evidence; 4 studies, 765 participants) and, again, insufficient evidence to determine whether rates of SAEs differed between groups (RR 0.89, 95% CI 0.59 to 1.34; I2 = 23%; 10 studies, 3263 participants; very low‐certainty evidence).
Results from the NMA were consistent with those from pairwise meta‐analyses for all critical outcomes, and there was no indication of inconsistency within the networks.
Data from non‐randomized studies were consistent with RCT data. The most commonly reported AEs were throat/mouth irritation, headache, cough, and nausea, which tended to dissipate with continued EC use. Very few studies reported data on other outcomes or comparisons, hence, evidence for these is limited, with CIs often encompassing both clinically significant harm and benefit.
Authors’ conclusions
There is high‐certainty evidence that ECs with nicotine increase quit rates compared to NRT and moderate‐certainty evidence that they increase quit rates compared to ECs without nicotine. Evidence comparing nicotine EC with usual care/no treatment also suggests benefit, but is less certain due to risk of bias inherent in the study design. Confidence intervals were for the most part wide for data on AEs, SAEs and other safety markers, with no difference in AEs between nicotine and non‐nicotine ECs nor between nicotine ECs and NRT. Overall incidence of SAEs was low across all study arms. We did not detect evidence of serious harm from nicotine EC, but the longest follow‐up was two years and the number of studies was small.
The main limitation of the evidence base remains imprecision due to the small number of RCTs, often with low event rates. Further RCTs are underway. To ensure the review continues to provide up‐to‐date information to decision‐makers, this review is a living systematic review. We run searches monthly, with the review updated when relevant new evidence becomes available. Please refer to the Cochrane Database of Systematic Reviews for the review’s current status.