2024-01-08 タフツ大学
◆彼女の研究室はADAM8が新しい血管形成を助け、がんを成長させることがわかり、その抗体治療法の開発を進めている。ADAM8の活性は他のがんでも見られ、この治療法が多くのがん患者に影響を与える可能性がある。
<関連情報>
- https://now.tufts.edu/2024/01/08/turning-basic-research-new-treatment-most-aggressive-forms-breast-cancer
- https://cancerci.biomedcentral.com/articles/10.1186/s12935-023-03024-3
ADAM8は乳癌に広く発現し、ホルモン受容体陽性、HER-2陰性患者の予後不良を予測する ADAM8 is expressed widely in breast cancer and predicts poor outcome in hormone receptor positive, HER-2 negative patients
Stefania Pianetti,Kathy D. Miller,Hannah H. Chen,Sandra Althouse,Sha Cao,Steven J. Michael,Gail E. Sonenshein & Nora D. Mineva
Cancer Cell International Published:11 August 2023
DOI:https://doi.org/10.1186/s12935-023-03024-3
Abstract
Background
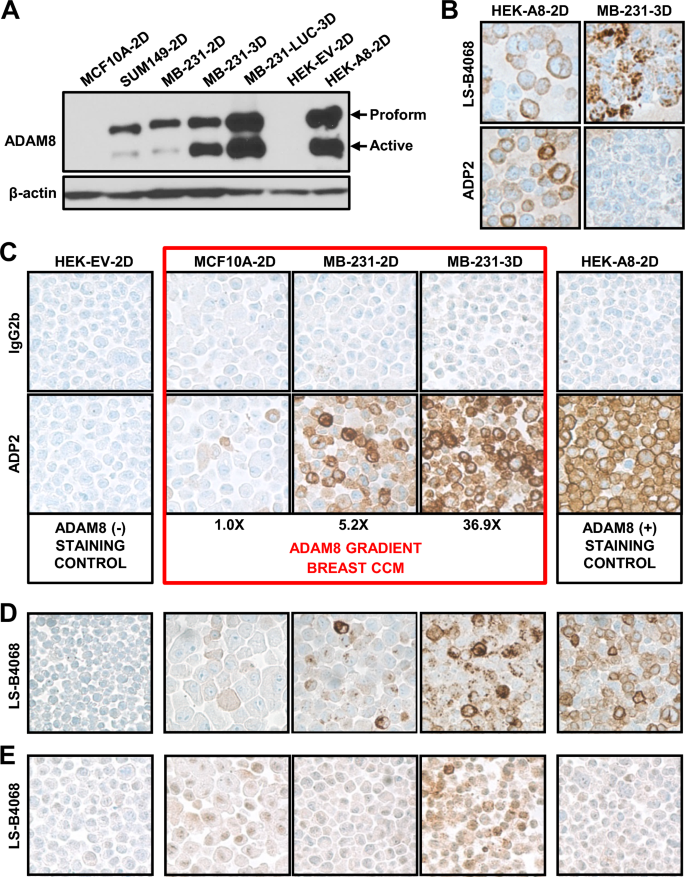
Breast malignancies are the predominant cancer-related cause of death in women. New methods of diagnosis, prognosis and treatment are necessary. Previously, we identified the breast cancer cell surface protein ADAM8 as a marker of poor survival, and a driver of Triple-Negative Breast Cancer (TNBC) growth and spread. Immunohistochemistry (IHC) with a research-only anti-ADAM8 antibody revealed 34.0% of TNBCs (17/50) expressed ADAM8. To identify those patients who could benefit from future ADAM8-based interventions, new clinical tests are needed. Here, we report on the preclinical development of a highly specific IHC assay for detection of ADAM8-positive breast tumors.
Methods
Formalin-fixed paraffin-embedded sections of ADAM8-positive breast cell lines and patient-derived xenograft tumors were used in IHC to identify a lead antibody, appropriate staining conditions and controls. Patient breast cancer samples (n = 490) were used to validate the assay. Cox proportional hazards models assessed association between survival and ADAM8 expression.
Results
ADAM8 staining conditions were optimized, a lead anti-human ADAM8 monoclonal IHC antibody (ADP2) identified, and a breast staining/scoring control cell line microarray (CCM) generated expressing a range of ADAM8 levels. Assay specificity, reproducibility, and appropriateness of the CCM for scoring tumor samples were demonstrated. Consistent with earlier findings, 36.1% (22/61) of patient TNBCs expressed ADAM8. Overall, 33.9% (166/490) of the breast cancer population was ADAM8-positive, including Hormone Receptor (HR) and Human Epidermal Growth Factor Receptor-2 (HER2) positive cancers, which were tested for the first time. For the most prevalent HR-positive/HER2-negative subtype, high ADAM8 expression identified patients at risk of poor survival.
Conclusions
Our studies show ADAM8 is widely expressed in breast cancer and provide support for both a diagnostic and prognostic value of the ADP2 IHC assay. As ADAM8 has been implicated in multiple solid malignancies, continued development of this assay may have broad impact on cancer management.