2024-01-16 韓国基礎科学研究院(IBS)
◆11C-アセテートPETイメージングが、GBMの診断と手術切除領域の特定に有効であることが示されました。また、アストロサイトのアセテート代謝を治療に活かす可能性も検討され、アストロサイトが腫瘍微小環境のエネルギー源としてアセテートを利用していることが判明しました。
◆研究チームは、反応性アストロサイトがGBMの治療の有望なターゲットであり、「Theragnosis」の観点から11C-アセテートPETイメージングと治療を組み合わせる重要性を強調しています。
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do
- https://academic.oup.com/neuro-oncology/advance-article-abstract/doi/10.1093/neuonc/noad243/7471337
膠芽腫腫瘍微小環境の反応性アストロサイトにおけるMCT1を介したがん由来の酢酸取り込みの可視化 Visualizing Cancer-Originating Acetate Uptake Through MCT1 in Reactive Astrocytes in the Glioblastoma Tumor Microenvironment
Dongwoo Kim, Hae Young Ko, Jee-In Chung, Yongmin Mason Park, Sangwon Lee, Seon Yoo Kim, Jisu Kim, Joong-Hyun Chun, Kyung-Seok Han, Misu Lee,Yeon Ha Ju, Sun Jun Park, Ki Duk Park, Min-Ho Nam, Se Hoon Kim, Jin-Kyoung Shim, Youngjoo Park, Hyunkeong Lim, Jaekyung Park, Hyunjin Kim, Suhyun Kim, Uiyeol Park, Hoon Ryu, So Yun Lee, Sunghyouk Park, Seok-Gu Kang, Jong Hee Chang, C Justin Lee, Mijin Yun
Neuro-oncology Published:12 December 2023
DOI:https://doi.org/10.1093/neuonc/noad243
Graphical Abstract
Abstract
Background
Reactive astrogliosis is a hallmark of various brain pathologies, including neurodegenerative diseases and glioblastomas. However, the specific intermediate metabolites contributing to reactive astrogliosis remain unknown. This study investigated how glioblastomas induce reactive astrogliosis in the neighboring microenvironment and explores 11C-acetate PET as an imaging technique for detecting reactive astrogliosis.
Methods
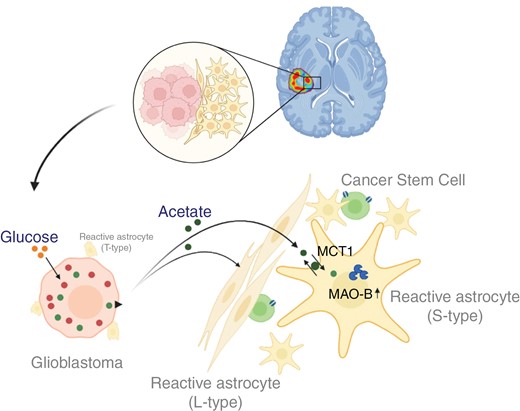
Through in vitro, mouse models, and human tissue experiments, we examined the association between elevated 11C-acetate uptake and reactive astrogliosis in gliomas. We explored acetate from glioblastoma cells, which triggers reactive astrogliosis in neighboring astrocytes by upregulating MAO-B and MCT1 expression. We evaluated the presence of cancer stem cells in the reactive astrogliosis region of glioblastomas and assessed the correlation between the volume of 11C-acetate uptake beyond MRI and prognosis.
Results
Elevated 11C-acetate uptake is associated with reactive astrogliosis and astrocytic MCT1 in the periphery of glioblastomas in human tissues and mouse models. Glioblastoma cells exhibit increased acetate production as a result of glucose metabolism, with subsequent secretion of acetate. Acetate derived from glioblastoma cells induces reactive astrogliosis in neighboring astrocytes by increasing the expression of MAO-B and MCT1. We found cancer stem cells within the reactive astrogliosis at the tumor periphery. Consequently, a larger volume of 11C-acetate uptake beyond contrast-enhanced MRI was associated with worse prognosis.
Conclusion
Our results highlight the role of acetate derived from glioblastoma cells in inducing reactive astrogliosis and underscore the potential value of 11C-acetate PET as an imaging technique for detecting reactive astrogliosis, offering important implications for the diagnosis and treatment of glioblastomas.


