2024-01-31 マックス・プランク研究所
◆Mcf1の構造はシーホースに似ており、トキシンが昆虫の宿主に放出されると、複数の有毒なペイロードを持つ頭部があり、尾部が目標細胞にアタッチします。このトキシンは、宿主昆虫内で細胞死を引き起こし、将来的には新しい生物農薬や昆虫制御に役立つトキシン変異の開発に寄与する可能性があります。
<関連情報>
- https://www.mpg.de/21490354/0131-moph-identify-engage-assassinate-how-seahorse-like-toxins-kill-insects-151445-x
- https://www.nature.com/articles/s41564-023-01571-z
- https://www.nature.com/articles/s41467-023-44069-2
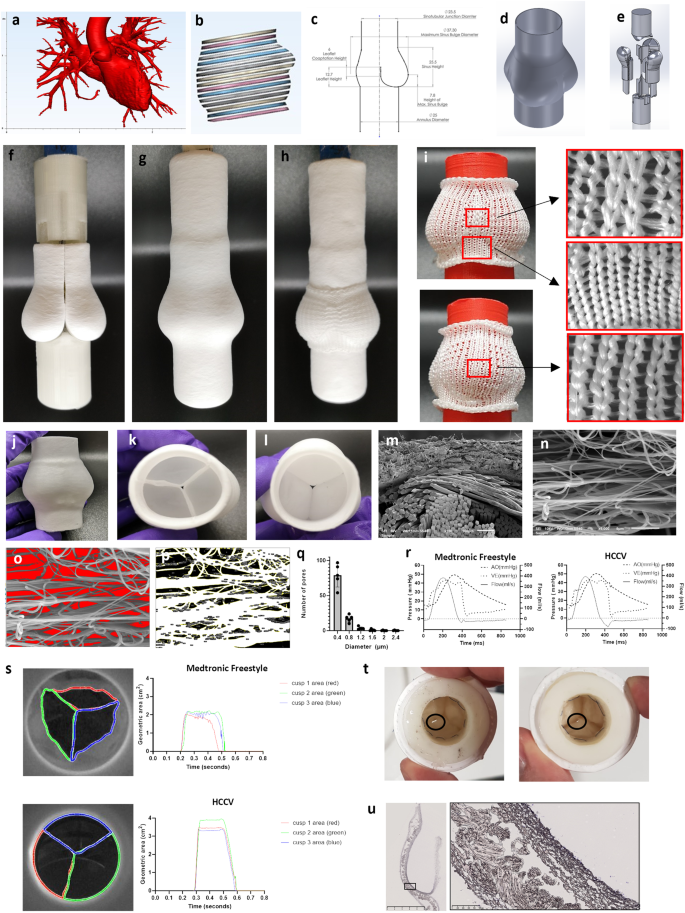
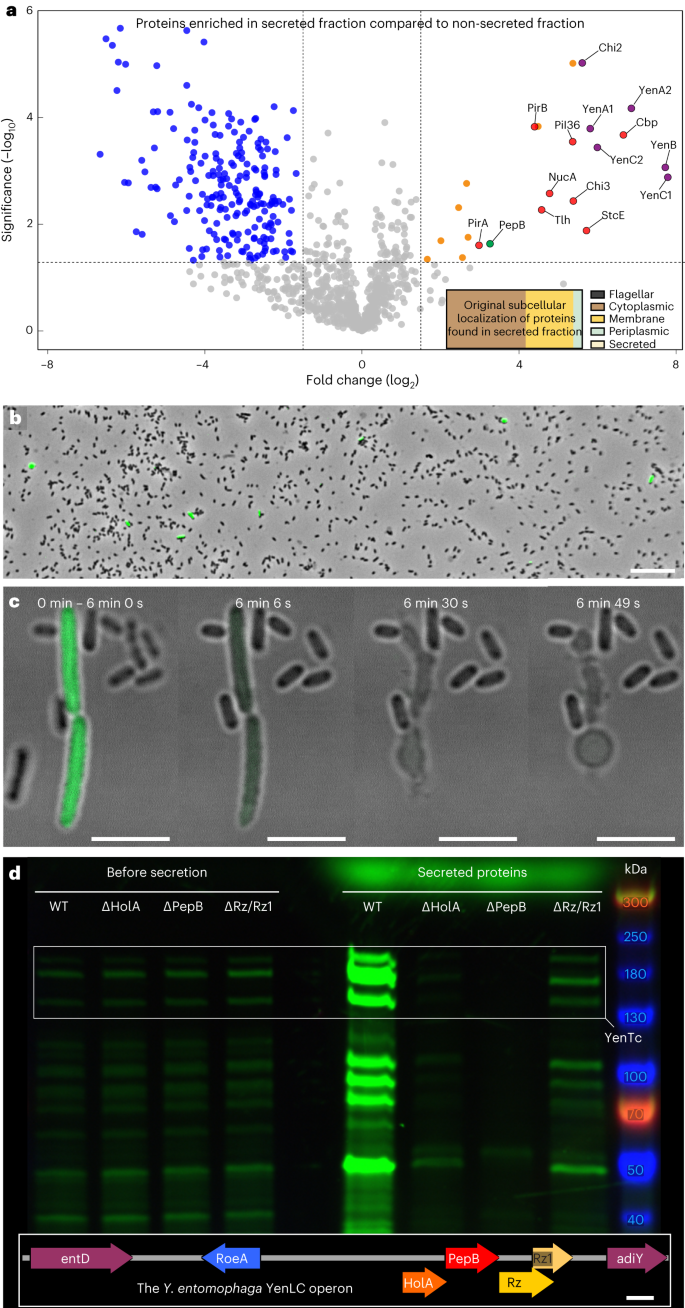
エルシニア・エントモファーガTc毒素はT10SS依存的に特殊化した細胞亜集団を溶解することで放出される Yersinia entomophaga Tc toxin is released by T10SS-dependent lysis of specialized cell subpopulations
Oleg Sitsel,Zhexin Wang,Petra Janning,Lara Kroczek,Thorsten Wagner & Stefan Raunser
Nature Microbiology Published:18 January 2024
DOI:https://doi.org/10.1038/s41564-023-01571-z
Abstract
Disease-causing bacteria secrete numerous toxins to invade and subjugate their hosts. Unlike many smaller toxins, the secretion machinery of most large toxins remains enigmatic. By combining genomic editing, proteomic profiling and cryo-electron tomography of the insect pathogen Yersinia entomophaga, we demonstrate that a specialized subset of these cells produces a complex toxin cocktail, including the nearly ribosome-sized Tc toxin YenTc, which is subsequently exported by controlled cell lysis using a transcriptionally coupled, pH-dependent type 10 secretion system (T10SS). Our results dissect the Tc toxin export process by a T10SS, identifying that T10SSs operate via a previously unknown lytic mode of action and establishing them as crucial players in the size-insensitive release of cytoplasmically folded toxins. With T10SSs directly embedded in Tc toxin operons of major pathogens, we anticipate that our findings may model an important aspect of pathogenesis in bacteria with substantial impact on agriculture and healthcare.
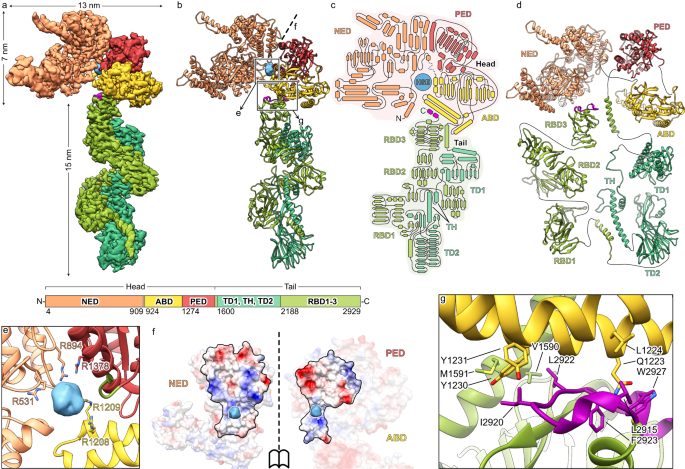
メイクス毛虫のフロッピー1毒素の構造と活性化機構 Structure and activation mechanism of the Makes caterpillars floppy 1 toxin
Alexander Belyy,Philipp Heilen,Philine Hagel,Oliver Hofnagel & Stefan Raunser
Nature Communications Published:12 December 2023
DOI:https://doi.org/10.1038/s41467-023-44069-2
Abstract
The bacterial Makes caterpillars floppy 1 (Mcf1) toxin promotes apoptosis in insects, leading to loss of body turgor and death. The molecular mechanism underlying Mcf1 intoxication is poorly understood. Here, we present the cryo-EM structure of Mcf1 from Photorhabdus luminescens, revealing a seahorse-like shape with a head and tail. While the three head domains contain two effectors, as well as an activator-binding domain (ABD) and an autoprotease, the tail consists of two putative translocation and three putative receptor-binding domains. Rearrangement of the tail moves the C-terminus away from the ABD and allows binding of the host cell ADP-ribosylation factor 3, inducing conformational changes that position the cleavage site closer to the protease. This distinct activation mechanism that is based on a hook-loop interaction results in three autocleavage reactions and the release of two toxic effectors. Unexpectedly, the BH3-like domain containing ABD is not an active effector. Our findings allow us to understand key steps of Mcf1 intoxication at the molecular level.