2024-02-20 マサチューセッツ大学アマースト校
◆この研究は、2型糖尿病の遺伝性に関する理解を深めるもので、8つの異なる遺伝的変異の機構クラスターと疾患との関連性を特定しました。これにより、個々のクラスターと糖尿病合併症の関連が明らかになりました。糖尿病のメカニズムをよりよく理解することで、個々のリスクを予測し、早期の介入が可能になることが期待されます。
<関連情報>
- https://www.umass.edu/news/article/newly-discovered-genetic-markers-help-pinpoint-diabetes-risks-complications
- https://www.nature.com/articles/s41586-024-07019-6
2型糖尿病の病態生理学における不均一性の遺伝的要因 Genetic drivers of heterogeneity in type 2 diabetes pathophysiology
Ken Suzuki,Konstantinos Hatzikotoulas,Lorraine Southam,Henry J. Taylor,Xianyong Yin,Kim M. Lorenz,Ravi Mandla,Alicia Huerta-Chagoya,Giorgio E. M. Melloni,Stavroula Kanoni,Nigel W. Rayner,Ozvan Bocher,Ana Luiza Arruda,Kyuto Sonehara,Shinichi Namba,Simon S. K. Lee,Michael H. Preuss,Lauren E. Petty,Philip Schroeder,Brett Vanderwerff,Mart Kals,Fiona Bragg,Kuang Lin,Xiuqing Guo,VA Million Veteran Program,… Eleftheria Zeggini
Nature Published:19 February 2024
DOI:https://doi.org/10.1038/s41586-024-07019-6
Abstract
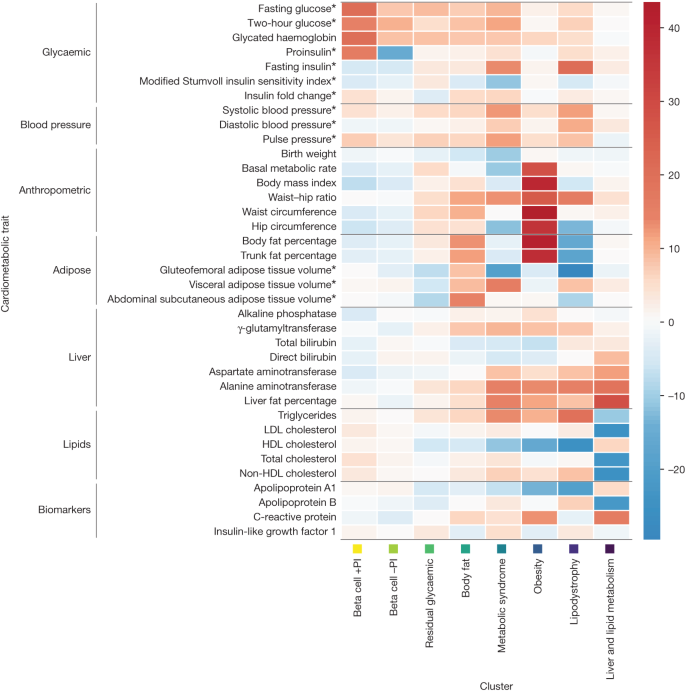
Type 2 diabetes (T2D) is a heterogeneous disease that develops through diverse pathophysiological processes1,2 and molecular mechanisms that are often specific to cell type3,4. Here, to characterize the genetic contribution to these processes across ancestry groups, we aggregate genome-wide association study data from 2,535,601 individuals (39.7% not of European ancestry), including 428,452 cases of T2D. We identify 1,289 independent association signals at genome-wide significance (P < 5 × 10-8) that map to 611 loci, of which 145 loci are, to our knowledge, previously unreported. We define eight non-overlapping clusters of T2D signals that are characterized by distinct profiles of cardiometabolic trait associations. These clusters are differentially enriched for cell-type-specific regions of open chromatin, including pancreatic islets, adipocytes, endothelial cells and enteroendocrine cells. We build cluster-specific partitioned polygenic scores5 in a further 279,552 individuals of diverse ancestry, including 30,288 cases of T2D, and test their association with T2D-related vascular outcomes. Cluster-specific partitioned polygenic scores are associated with coronary artery disease, peripheral artery disease and end-stage diabetic nephropathy across ancestry groups, highlighting the importance of obesity-related processes in the development of vascular outcomes. Our findings show the value of integrating multi-ancestry genome-wide association study data with single-cell epigenomics to disentangle the aetiological heterogeneity that drives the development and progression of T2D. This might offer a route to optimize global access to genetically informed diabetes care.