2022-03-25 スウェーデン王・王立工科大学(KTH)
・本論文の第一の目的は、MSにおける神経変性の定量的計測法を、脳梁に着目して評価・開発することである。これは、手作業による描出、従来の体積測定法、機械学習アプローチの精度と正確さの比較を通じて達成された。
- https://www.kth.se/en/om/mot/kalender/quantitative-mri-biomarkers-of-neurodegeneration-in-multiple-sclerosis-1.1145656?date=2022-03-25&orgdate=2022-03-25&length=1&orglength=0
- http://kth.diva-portal.org/smash/get/diva2:1639434/FULLTEXT03.pdf
- http://kth.diva-portal.org/smash/record.jsf?pid=diva2%3A1639434&dswid=7946
定量的MRIバイオマーカー多発性硬化症における神経変性について Quantitative MRI Biomarkers of Neurodegeneration in Multiple Sclerosis
Maikeru puratten
Published:2022-03-25
Abstract
Background: Multiple sclerosis (MS) is a chronic neuroinflammatory and neurodegenerative disease that targets myelin in the brain and spinal cord. The corpus callosum connects the cerebral hemispheres and is composed of heavily myelinated axons. Atrophy of the corpus callosum has been explored as a more sensitive marker of disease status and neurodegeneration relative to other neuroanatomical structures. However, development of more accurate, precise and less labor demanding tools for characterizing callosal atrophy would increase its potential as a proxy marker of MS evolution.
Purpose: The primary objective of this thesis was to evaluate and develop quantitative methods for measuring neurodegeneration in MS with a focus on the corpus callosum. This was achieved through the comparison of the accuracy and precision of manual delineation, conventional volumetric methods, and machine learning approaches.
Study I: In a prospective study, 9 MS patients underwent scan/re-scanning with and without repositioning to measure the precision and accuracy of manual versus volumetric cross-sectional and longitudinal FreeSurfer analyses. While the longitudinal stream of FreeSurfer revealed the highest precision, the overall limitations on accuracy warrants caution.
Study II: In a prospective study, 553 MS patients with 704 2D T2-weighted MRI acquisitions were used to train and validate a machine learning algorithm for segmenting a marker of neurodegeneration. The algorithm quickly produced highly accurate segmentations of the corpus callosum and brain (Dice Coefficient: 89% and 98%, respectively). The algorithm had numerically higher correlations to neurologic disability as compared to FreeSurfer.
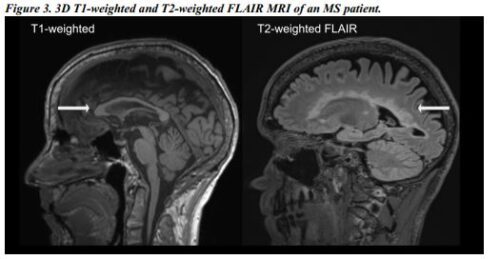
Study III: Analogous to Study II, in a prospective study, 631 MS patients with 3D T1-weighted and T2-weighted FLAIR acquisitions were used to train and validate a machine learning algorithm for segmenting the mid-sagittal normalized corpus callosum area. The algorithm performed better with T1-weighted scans and less atrophied patients. Scanner parameters had no significant effect on the T1-weighted output. The algorithm produced segmentations in less than a minute per scan, with similar correlations to neurologic disability, as compared to FreeSurfer.
Study IV: In a prospective study, 92 MS patients acquired both 3 and 7 Tesla brain MRI scans to reveal the lobe-specific lesion volumes’ association to corpus callosum atrophy, where lesion burden was found to be greatest in the frontal and parietal lobes. In addition, the posterior portions of the corpus callosum provided the strongest fit linear regression models, with a combination of white matter lesions and intracortical lesions predicting atrophy.
Conclusions: Creating and evaluating novel tools for measuring neurodegeneration over time is important both for monitoring disease progression and to evaluate therapeutic responses with current drugs. As novel therapeutic strategies appear, it may also help in assessing neuroregenerative approaches.