2024-02-20 カリフォルニア大学バークレー校(UCB)
◆鳥からのレトロトランスポゾンを利用した新しい技術は、遺伝子をヒトゲノムの「安全な港safe-harbor」に挿入し、基本的な遺伝子を妨げることなく癌を引き起こすことがないようにします。この新しいPRINT技術は、遺伝子挿入を簡素化し、CRISPR-Cas技術の補完的な能力として機能し、遺伝子療法の有望な方法です。
<関連情報>
- https://news.berkeley.edu/2024/02/20/junk-dna-in-birds-may-hold-key-to-safe-efficient-gene-therapy
- https://www.nature.com/articles/s41587-024-02137-y
- https://www.nature.com/articles/s41586-023-06933-5
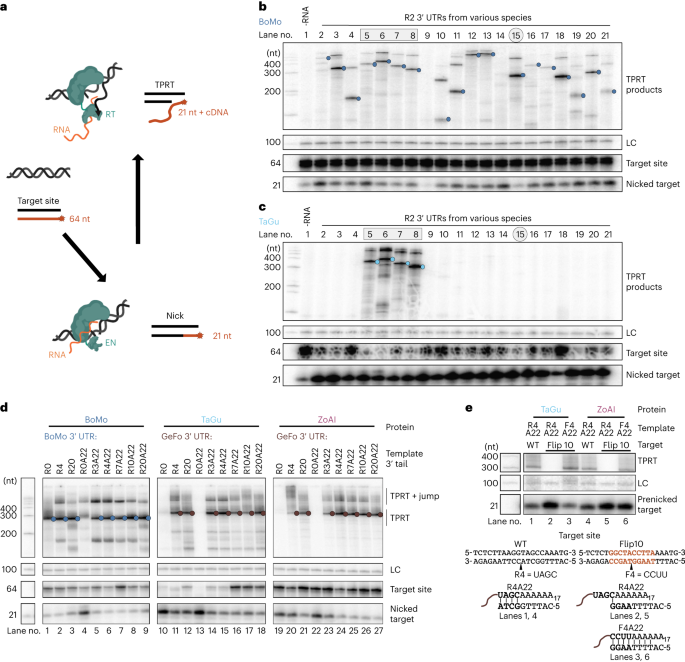
真核生物のレトロエレメントタンパク質を利用してヒトのセーフハーバー遺伝子座に導入遺伝子を挿入する Harnessing eukaryotic retroelement proteins for transgene insertion into human safe-harbor loci
Xiaozhu Zhang,Briana Van Treeck,Connor A. Horton,Jeremy J. R. McIntyre,Sarah M. Palm,Justin L. Shumate & Kathleen Collins
Nature Biotechnology Published:20 February 2024
DOI:https://doi.org/10.1038/s41587-024-02137-y
Abstract
Current approaches for inserting autonomous transgenes into the genome, such as CRISPR–Cas9 or virus-based strategies, have limitations including low efficiency and high risk of untargeted genome mutagenesis. Here, we describe precise RNA-mediated insertion of transgenes (PRINT), an approach for site-specifically primed reverse transcription that directs transgene synthesis directly into the genome at a multicopy safe-harbor locus. PRINT uses delivery of two in vitro transcribed RNAs: messenger RNA encoding avian R2 retroelement-protein and template RNA encoding a transgene of length validated up to 4 kb. The R2 protein coordinately recognizes the target site, nicks one strand at a precise location and primes complementary DNA synthesis for stable transgene insertion. With a cultured human primary cell line, over 50% of cells can gain several 2 kb transgenes, of which more than 50% are full-length. PRINT advantages include no extragenomic DNA, limiting risk of deleterious mutagenesis and innate immune responses, and the relatively low cost, rapid production and scalability of RNA-only delivery.
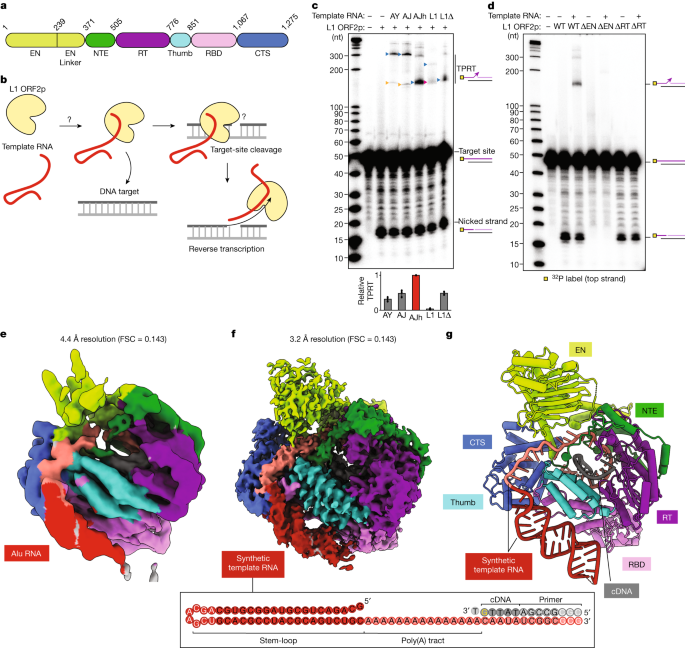
レトロトランスポジションにおけるヒトLINE-1による鋳型と標的部位の認識 Template and target-site recognition by human LINE-1 in retrotransposition
Akanksha Thawani,Alfredo Jose Florez Ariza,Eva Nogales & Kathleen Collins
Nature Published:14 December 2023
DOI:https://doi.org/10.1038/s41586-023-06933-5
Abstract
The long interspersed element-1 (LINE-1, hereafter L1) retrotransposon has generated nearly one-third of the human genome and serves as an active source of genetic diversity and human disease1. L1 spreads through a mechanism termed target-primed reverse transcription, in which the encoded enzyme (ORF2p) nicks the target DNA to prime reverse transcription of its own or non-self RNAs2. Here we purified full-length L1 ORF2p and biochemically reconstituted robust target-primed reverse transcription with template RNA and target-site DNA. We report cryo-electron microscopy structures of the complete human L1 ORF2p bound to structured template RNAs and initiating cDNA synthesis. The template polyadenosine tract is recognized in a sequence-specific manner by five distinct domains. Among them, an RNA-binding domain bends the template backbone to allow engagement of an RNA hairpin stem with the L1 ORF2p C-terminal segment. Moreover, structure and biochemical reconstitutions demonstrate an unexpected target-site requirement: L1 ORF2p relies on upstream single-stranded DNA to position the adjacent duplex in the endonuclease active site for nicking of the longer DNA strand, with a single nick generating a staggered DNA break. Our research provides insights into the mechanism of ongoing transposition in the human genome and informs the engineering of retrotransposon proteins for gene therapy.