2024-03-18 ワシントン州立大学(WSU)
 A natural serpentine soil outcrop — the area devoid of vegetation — indicates where high levels of the heavy metal, nickel, are too toxic for many plants to grow. Image taken at the Hopland Research and Extension Center, CA. Photo by Angeliqua Montoya, WSU
A natural serpentine soil outcrop — the area devoid of vegetation — indicates where high levels of the heavy metal, nickel, are too toxic for many plants to grow. Image taken at the Hopland Research and Extension Center, CA. Photo by Angeliqua Montoya, WSU
<関連情報>
- https://news.wsu.edu/press-release/2024/03/18/genes-identified-that-allow-bacteria-to-thrive-despite-toxic-heavy-metal-in-soil/
- https://www.pnas.org/doi/10.1073/pnas.2311127121
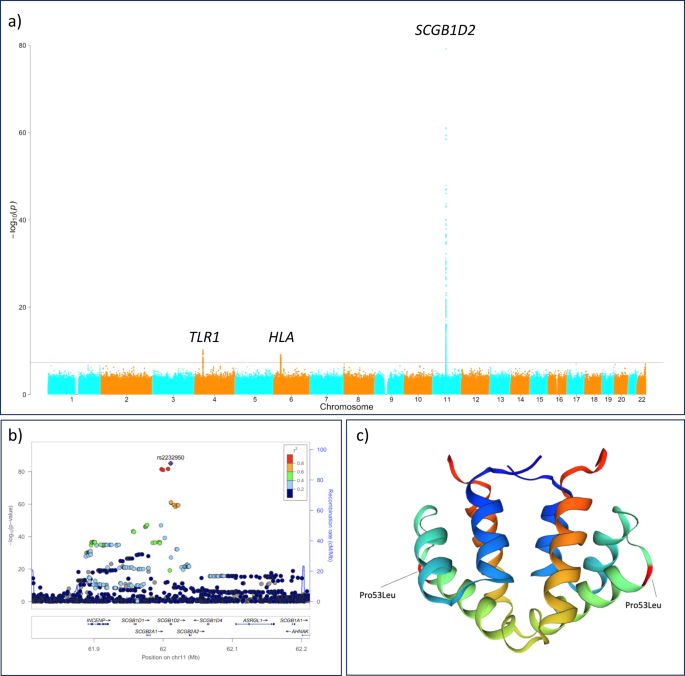
野生の根粒菌におけるストレス適応の進化ゲノミクス The evolutionary genomics of adaptation to stress in wild rhizobium bacteria
Hanna Kehlet-Delgado, Angeliqua P. Montoya, Kyson T. Jensen, +6, and Stephanie S. Porter
Proceedings of the National Academy of Sciences Published:March 20, 2024
DOI:https://doi.org/10.1073/pnas.2311127121
Significance
There is great interest in investigating the genetic basis for adaptation in microbes, yet few studies reveal both the genes and evolutionary dynamics that allow microbes to adapt to natural environmental variation. We identify genes associated with the ability to tolerate stressful soil conditions in wild symbiotic bacteria and demonstrate that these genes drive replicated patterns of adaptation across the landscape. Phylogenetic evidence indicates that these adaptive genes are transferred among otherwise distinct lineages and drive parallel molecular solutions to stress among populations across large spatial scales. These findings reveal molecular processes of adaptation in wild microbes across the landscape and are widely applicable to efforts to understand the evolutionary origins of microbial diversity.
Abstract
Microbiota comprise the bulk of life’s diversity, yet we know little about how populations of microbes accumulate adaptive diversity across natural landscapes. Adaptation to stressful soil conditions in plants provides seminal examples of adaptation in response to natural selection via allelic substitution. For microbes symbiotic with plants however, horizontal gene transfer allows for adaptation via gene gain and loss, which could generate fundamentally different evolutionary dynamics. We use comparative genomics and genetics to elucidate the evolutionary mechanisms of adaptation to physiologically stressful serpentine soils in rhizobial bacteria in western North American grasslands. In vitro experiments demonstrate that the presence of a locus of major effect, the nre operon, is necessary and sufficient to confer adaptation to nickel, a heavy metal enriched to toxic levels in serpentine soil, and a major axis of environmental soil chemistry variation. We find discordance between inferred evolutionary histories of the core genome and nreAXY genes, which often reside in putative genomic islands. This suggests that the evolutionary history of this adaptive variant is marked by frequent losses, and/or gains via horizontal acquisition across divergent rhizobium clades. However, different nre alleles confer distinct levels of nickel resistance, suggesting allelic substitution could also play a role in rhizobium adaptation to serpentine soil. These results illustrate that the interplay between evolution via gene gain and loss and evolution via allelic substitution may underlie adaptation in wild soil microbiota. Both processes are important to consider for understanding adaptive diversity in microbes and improving stress-adapted microbial inocula for human use.