2024-07-12 バッファロー大学(UB)
<関連情報>
- https://www.buffalo.edu/news/releases/2024/07/study-reveals-how-liquid-protein-droplets-age-into-rubber-ball-like-elastic-solids.html
- https://www.nature.com/articles/s41567-024-02558-1
配列特異的相互作用がタンパク質凝縮体の粘弾性と老化ダイナミクスを決定する Sequence-specific interactions determine viscoelasticity and ageing dynamics of protein condensates
Ibraheem Alshareedah,Wade M. Borcherds,Samuel R. Cohen,Anurag Singh,Ammon E. Posey,Mina Farag,Anne Bremer,Gregory W. Strout,Dylan T. Tomares,Rohit V. Pappu,Tanja Mittag & Priya R. Banerjee
Nature Physics Published:02 July 2024
DOI:https://doi.org/10.1038/s41567-024-02558-1
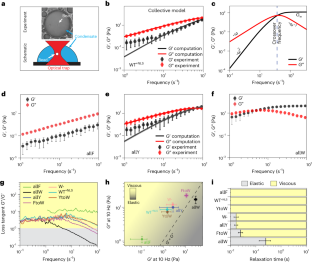
Abstract
Biomolecular condensates are viscoelastic materials. Here we investigate the determinants of the sequence-encoded and age-dependent viscoelasticity of condensates formed by the prion-like low-complexity domain of the protein hnRNP A1 and its designed variants. We find that the dominantly viscous forms of the condensates are metastable Maxwell fluids. A Rouse–Zimm model that accounts for the network-like organization of proteins within condensates reproduces the measured viscoelastic moduli. We show that the strengths of aromatic inter-sticker interactions determine sequence-specific amplitudes of elastic and viscous moduli and the timescales over which elastic properties dominate. These condensates undergo physical ageing on sequence-specific timescales. This is driven by mutations to spacer residues that weaken the metastability of dominantly viscous phases. The ageing of condensates is accompanied by disorder-to-order transitions, leading to the formation of non-fibrillar, β-sheet-containing, semi-crystalline, elastic, Kelvin–Voigt solids. Our results suggest that sequence grammars, which refer to amino acid identities of stickers versus spacers in prion-like low-complexity domains, have evolved to afford control over metastabilities of dominantly viscous fluid phases of condensates. This selection is likely to render barriers for conversion from metastable fluids to globally stable solids insurmountable on functionally relevant timescales.

