2024-07-26 ノースウェスタン大学
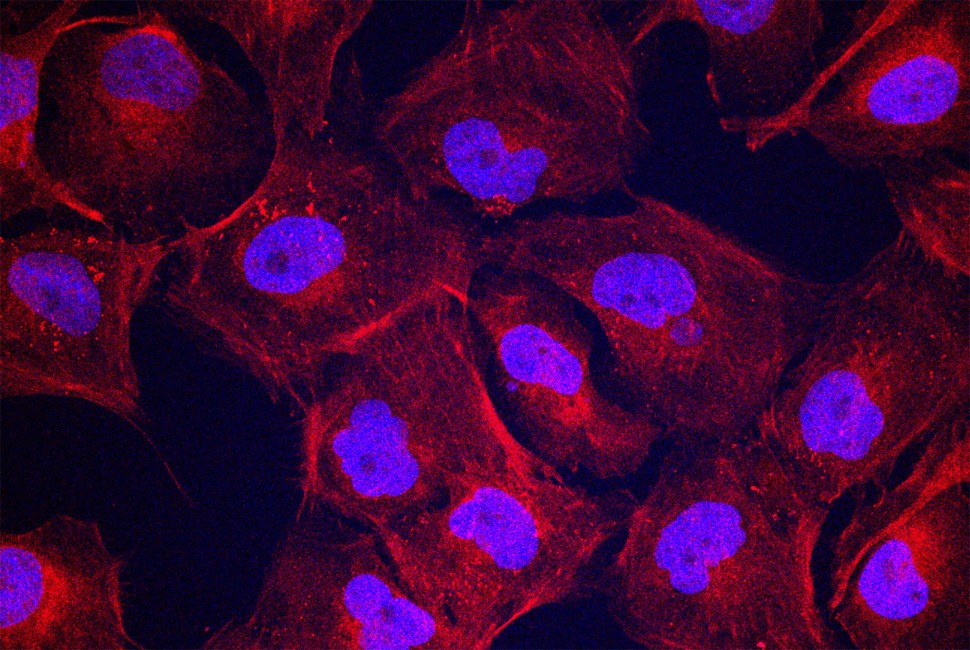
In new experiments, human cartilage cells treated with fast-moving dancing molecules made more collagen II (shown in red), a crucial component for regeneration. Cell nuclei are shown in blue/purple. Credit: Stupp Research Group/Northwestern
この治療法は、今回の研究で人間の軟骨細胞にも適用され、わずか4時間で軟骨再生に必要な遺伝子発現を活性化しました。さらに、3日後には軟骨再生に必要なタンパク質成分が生成されました。研究は、分子の「ダンス」運動が治療効果を高めることを示しました。この発見は軟骨再生にとどまらず、将来的には他の組織再生にも応用できる可能性があります。
<関連情報>
- https://news.northwestern.edu/stories/2024/july/dancing-molecules-heal-cartilage-damage/
- https://pubs.acs.org/doi/10.1021/jacs.4c05170
- https://www.science.org/doi/10.1126/science.abh3602
超分子運動がトランスフォーミング成長因子β1の環状ペプチド模倣体の軟骨生物活性を可能にする Supramolecular Motion Enables Chondrogenic Bioactivity of a Cyclic Peptide Mimetic of Transforming Growth Factor-β1
Shelby C. Yuan,Zaida Álvarez,Sieun Ruth Lee,Radoslav Z. Pavlović,Chunhua Yuan,Ethan Singer,Steven J. Weigand,Liam C. Palmer,Samuel I. Stupp
Journal of the American Chemical Published: July 25, 2024
DOI:https://doi.org/10.1021/jacs.4c05170
Abstract

Transforming growth factor (TGF)-β1 is a multifunctional protein that is essential in many cellular processes that include fibrosis, inflammation, chondrogenesis, and cartilage repair. In particular, cartilage repair is important to avoid physical disability since this tissue does not have the inherent capacity to regenerate beyond full development. We report here on supramolecular coassemblies of two peptide amphiphile molecules, one containing a TGF-β1 mimetic peptide, and another which is one of two constitutional isomers lacking bioactivity. Using human articular chondrocytes, we investigated the bioactivity of the supramolecular copolymers of each isomer displaying either the previously reported linear form of the mimetic peptide or a novel cyclic analogue. Based on fluorescence depolarization and 1H NMR spin–lattice relaxation times, we found that coassemblies containing the cyclic compound and the most dynamic isomer exhibited the highest intracellular TGF-β1 signaling and gene expression of cartilage extracellular matrix components. We conclude that control of supramolecular motion is emerging as an important factor in the binding of synthetic molecules to receptors that can be tuned through chemical structure.
超分子運動を強化した生物活性足場が脊髄損傷からの回復を促進する Bioactive scaffolds with enhanced supramolecular motion promote recovery from spinal cord injury
Z. Álvarez, A. N. Kolberg-Edelbrock, I. R. Sasselli, J. A. Ortega, […], and S. I. Stupp
Science Published:11 Nov 2021
DOI:https://doi.org/10.1126/science.abh3602

Fibril motion improves peptide signaling
Artificial scaffolds that bear the peptide-signaling sequences of proteins for tissue regeneration often have limited effectiveness. Álvarez et al. synthesized supramolecular peptide fibril scaffolds bearing two peptide sequences that promote nerve regeneration, one that reduces glial scarring and another that promotes blood vessel formation (see the Perspective by Wojciechowski and Stevens). In a mouse model of paralyzing human spinal cord injury, mutations in a tetrapeptide domain outside of the signaling regions improved recovery by promoting intense supramolecular motion within the fibrils. The mutation with the most intense dynamics resulted in corticospinal axon regrowth and myelination, functional revascularization, and motor neuron survival. —PDS
Abstract
The signaling of cells by scaffolds of synthetic molecules that mimic proteins is known to be effective in the regeneration of tissues. Here, we describe peptide amphiphile supramolecular polymers containing two distinct signals and test them in a mouse model of severe spinal cord injury. One signal activates the transmembrane receptor β1-integrin and a second one activates the basic fibroblast growth factor 2 receptor. By mutating the peptide sequence of the amphiphilic monomers in nonbioactive domains, we intensified the motions of molecules within scaffold fibrils. This resulted in notable differences in vascular growth, axonal regeneration, myelination, survival of motor neurons, reduced gliosis, and functional recovery. We hypothesize that the signaling of cells by ensembles of molecules could be optimized by tuning their internal motions.

