2024-08-28 カリフォルニア工科大学(Caltech)
カリフォルニア工科大学や他の研究機関のチームが、タンパク質生成の重要な調整役である小さなシャペロンタンパク質複合体NACの役割を明らかにしました。NACはリボソームで新たに生成されたタンパク質の初期修飾を調整し、約40%のタンパク質に影響を与えます。具体的には、タンパク質の最初のアミノ酸であるメチオニンの除去とアセチル基の付加を制御します。これらの修飾はタンパク質の機能や疾患の発生に関わる重要な過程です。
<関連情報>
- https://www.caltech.edu/about/news/a-master-regulator-of-protein-production
- https://www.nature.com/articles/s41586-024-07846-7
NACは新生タンパク質プロセッシングのためにリボソーム多酵素複合体を誘導する NAC guides a ribosomal multienzyme complex for nascent protein processing
Alfred M. Lentzsch,Denis Yudin,Martin Gamerdinger,Sowmya Chandrasekar,Laurenz Rabl,Alain Scaiola,Elke Deuerling,Nenad Ban & Shu-ou Shan
Nature Published:21 August 2024
DOI:https://doi.org/10.1038/s41586-024-07846-7
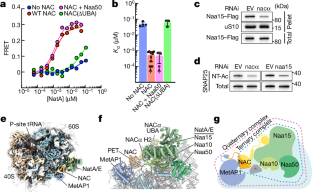
Abstract
Approximately 40% of the mammalian proteome undergoes N-terminal methionine excision and acetylation, mediated sequentially by methionine aminopeptidase (MetAP) and N-acetyltransferase A (NatA), respectively1. Both modifications are strictly cotranslational and essential in higher eukaryotic organisms1. The interaction, activity and regulation of these enzymes on translating ribosomes are poorly understood. Here we perform biochemical, structural and in vivo studies to demonstrate that the nascent polypeptide-associated complex2,3 (NAC) orchestrates the action of these enzymes. NAC assembles a multienzyme complex with MetAP1 and NatA early during translation and pre-positions the active sites of both enzymes for timely sequential processing of the nascent protein. NAC further releases the inhibitory interactions from the NatA regulatory protein huntingtin yeast two-hybrid protein K4,5 (HYPK) to activate NatA on the ribosome, enforcing cotranslational N-terminal acetylation. Our results provide a mechanistic model for the cotranslational processing of proteins in eukaryotic cells.

