2024-10-04 ゲーテ大学
<関連情報>
- https://aktuelles.uni-frankfurt.de/english/pharmacology-venomous-crustacean-from-mayan-underwater-caves-provides-new-drug-candidates/
- https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-024-01955-5
レミペデ毒から多様に進化したキシバルビン変異体はカリウムチャネルを阻害し、PKA-IIとErk1/2シグナル伝達を活性化する Diversely evolved xibalbin variants from remipede venom inhibit potassium channels and activate PKA-II and Erk1/2 signaling
Ernesto Lopes Pinheiro-Junior,Ehsan Alirahimi,Steve Peigneur,Jörg Isensee,Susanne Schiffmann,Pelin Erkoc,Robert Fürst,Andreas Vilcinskas,Tobias Sennoner,Ivan Koludarov,Benjamin-Florian Hempel,Jan Tytgat,Tim Hucho & Björn M. von Reumont
BMC Biology Published:29 July 2024
DOI:https://doi.org/10.1186/s12915-024-01955-5
Abstract
Background
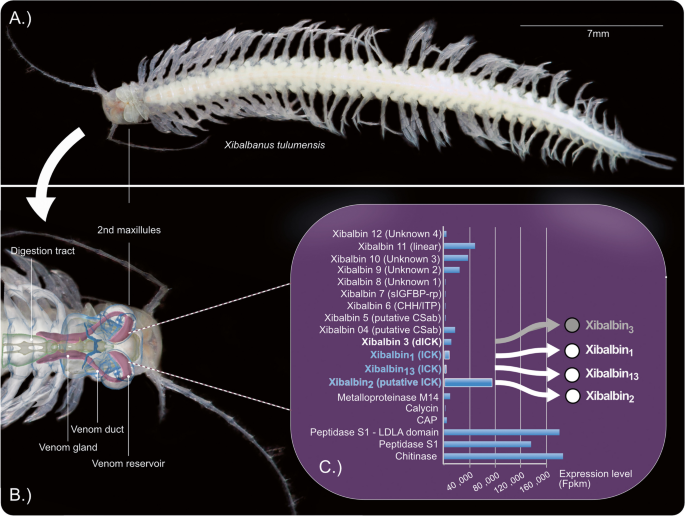
The identification of novel toxins from overlooked and taxonomically exceptional species bears potential for various pharmacological applications. The remipede Xibalbanus tulumensis, an underwater cave-dwelling crustacean, is the only crustacean for which a venom system has been described. Its venom contains several xibalbin peptides that have an inhibitor cysteine knot (ICK) scaffold.
Results
Our screenings revealed that all tested xibalbin variants particularly inhibit potassium channels. Xib1 and xib13 with their eight-cysteine domain similar to spider knottins also inhibit voltage-gated sodium channels. No activity was noted on calcium channels. Expanding the functional testing, we demonstrate that xib1 and xib13 increase PKA-II and Erk1/2 sensitization signaling in nociceptive neurons, which may initiate pain sensitization. Our phylogenetic analysis suggests that xib13 either originates from the common ancestor of pancrustaceans or earlier while xib1 is more restricted to remipedes. The ten-cysteine scaffolded xib2 emerged from xib1, a result that is supported by our phylogenetic and machine learning-based analyses.
Conclusions
Our functional characterization of synthesized variants of xib1, xib2, and xib13 elucidates their potential as inhibitors of potassium channels in mammalian systems. The specific interaction of xib2 with Kv1.6 channels, which are relevant to treating variants of epilepsy, shows potential for further studies. At higher concentrations, xib1 and xib13 activate the kinases PKA-II and ERK1/2 in mammalian sensory neurons, suggesting pain sensitization and potential applications related to pain research and therapy. While tested insect channels suggest that all probably act as neurotoxins, the biological function of xib1, xib2, and xib13 requires further elucidation. A novel finding on their evolutionary origin is the apparent emergence of X. tulumensis-specific xib2 from xib1. Our study is an important cornerstone for future studies to untangle the origin and function of these enigmatic proteins as important components of remipede but also other pancrustacean and arthropod venoms.