2024-10-10 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/oxidative-stress-how-protein-recycling-protects-against-cell-death.html
- https://www.nature.com/articles/s41418-024-01398-z
DDI2によるNFE2L1-ユビキチン・プロテアソーム系の活性化がフェロプターシスを防ぐ Activating the NFE2L1-ubiquitin-proteasome system by DDI2 protects from ferroptosis
Anahita Ofoghi,Stefan Kotschi,Imke L. Lemmer,Daniel T. Haas,Nienke Willemsen,Batoul Bayer,Anna S. Jung,Sophie Möller,Stefanie Haberecht-Müller,Elke Krüger,Natalie Krahmer & Alexander Bartelt
Cell Death & Differentiation Published:09 October 2024
DOI:https://doi.org/10.1038/s41418-024-01398-z
Abstract
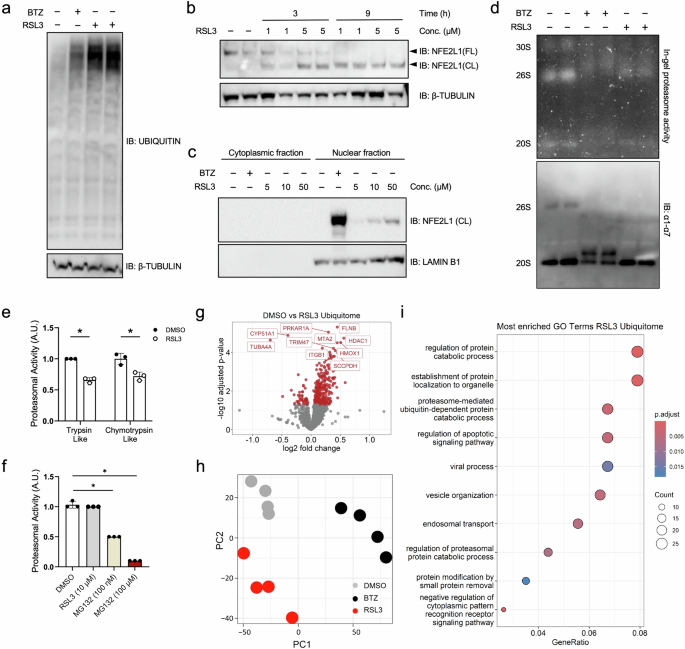
Ferroptosis is an iron-dependent, non-apoptotic form of cell death initiated by oxidative stress and lipid peroxidation. Recent evidence has linked ferroptosis to the action of the transcription factor Nuclear factor erythroid-2 derived,-like-1 (NFE2L1). NFE2L1 regulates proteasome abundance in an adaptive fashion, maintaining protein quality control to secure cellular homeostasis, but the regulation of NFE2L1 during ferroptosis and the role of the ubiquitin-proteasome system (UPS) herein are still unclear. In the present study, using an unbiased proteomic approach charting the specific ubiquitylation sites, we show that induction of ferroptosis leads to recalibration of the UPS. RSL3-induced ferroptosis inhibits proteasome activity and leads to global hyperubiquitylation, which is linked to NFE2L1 activation. As NFE2L1 resides in the endoplasmic reticulum tethered to the membrane, it undergoes complex posttranslational modification steps to become active and induce the expression of proteasome subunit genes. We show that proteolytic cleavage of NFE2L1 by the aspartyl protease DNA-damage inducible 1 homolog 2 (DDI2) is a critical step for the ferroptosis-induced feed-back loop of proteasome function. Cells lacking DDI2 cannot activate NFE2L1 in response to RSL3 and show global hyperubiquitylation. Genetic or chemical induction of ferroptosis in cells with a disrupted DDI2-NFE2L1 pathway diminishes proteasomal activity and promotes cell death. Also, treating cells with the clinical drug nelfinavir, which inhibits DDI2, sensitized cells to ferroptosis. In conclusion, our results provide new insight into the importance of the UPS in ferroptosis and highlight the role of the DDI2-NFE2L1 as a potential therapeutic target. Manipulating DDI2-NFE2L1 activity through chemical inhibition might help sensitizing cells to ferroptosis, thus enhancing existing cancer therapies.


