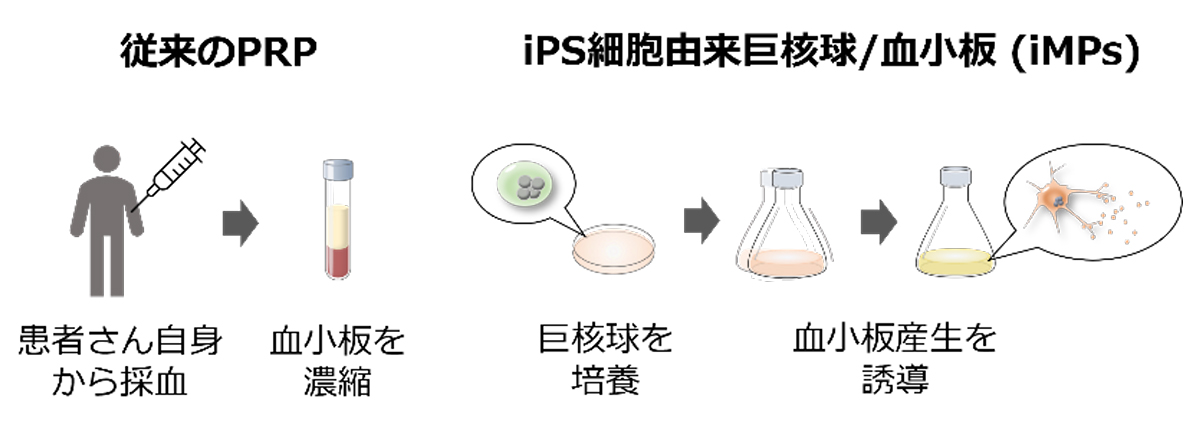
2024-11-22 インペリアル・カレッジ・ロンドン(ICL)
<関連情報>
- https://www.imperial.ac.uk/news/258534/genetic-clues-explain-children-develop-rare/
- https://rupress.org/jem/article/221/12/e920240699/277108/Heterozygous-BTNL8-variants-in-individuals-with
小児多系統炎症症候群(MIS-C)患者におけるBTNL8ヘテロ接合変異体
Heterozygous BTNL8 variants in individuals with multisystem inflammatory syndrome in children (MIS-C)
Evangelos Bellos , Dilys Santillo , Pierre Vantourout , Heather R. Jackson , Amedine Duret , Henry Hearn , Yoann Seeleuthner , Estelle Talouarn , Stephanie Hodeib , Harsita Patel , Oliver Powell , Sophya Yeoh , Sobia Mustafa , Dominic Habgood-Coote , Samuel Nichols , Leire Estramiana Elorrieta , Giselle D’Souza , Victoria J. Wright , Diego Estrada-Rivadeneyra , Adriana H. Tremoulet , Kirsten B. Dummer , Stejara A. Netea , Antonio Condino-Neto , Yu Lung Lau , Esmeralda Núñez Cuadros , Julie Toubiana , Marisol Holanda Pena , Frédéric Rieux-Laucat , Charles-Edouard Luyt , Filomeen Haerynck , Jean Louis Mège , Samya Chakravorty , Elie Haddad , Marie-Paule Morin , Özge Metin Akcan , Sevgi Keles , Melike Emiroglu , Gulsum Alkan , Sadiye Kübra Tüter Öz , Sefika Elmas Bozdemir , Guillaume Morelle , Alla Volokha , Yasemin Kendir-Demirkol , Betul Sözeri , Taner Coskuner , Aysun Yahsi, Belgin Gulhan , Saliha Kanik-Yuksek , Gulsum Iclal Bayhan , Aslinur Ozkaya-Parlakay , Osman Yesilbas , Nevin Hatipoglu , Tayfun Ozcelik , Alexandre Belot , Emilie Chopin , Vincent Barlogis , Esra Sevketoglu , Emin Menentoglu , Zeynep Gokce Gayretli Aydin , Marketa Bloomfield , Suzan A. AlKhater , Cyril Cyrus , Yuriy Stepanovskiy , Anastasiia Bondarenko , Fatma Nur Öz , Meltem Polat , Jiří Fremuth , Jan Lebl , Amyrath Geraldo , Emmanuelle Jouanguy , COVID-19 Human Genetic Effort, DIAMONDS, EUCLIDS, Michael J. Carter , Paul Wellman , Mark Peters , Rebeca Pérez de Diego , Lindsey Ann Edwards , Christopher Chiu , Mahdad Noursadeghi , Alexandre Bolze , Chisato Shimizu , Myrsini Kaforou , Melissa Shea Hamilton , Jethro A. Herberg , Erica G. Schmitt , Agusti Rodriguez-Palmero , Aurora Pujol , Jihoon Kim , Aurélie Cobat , Laurent Abel , Shen-Ying Zhang , Jean-Laurent Casanova , Taco W. Kuijpers , Jane C. Burns , Michael Levin , Adrian C. Hayday , Vanessa Sancho-Shimizu
Journal of Experimental Medicine Published:November 22 2024
DOI:https://doi.org/10.1084/jem.20240699
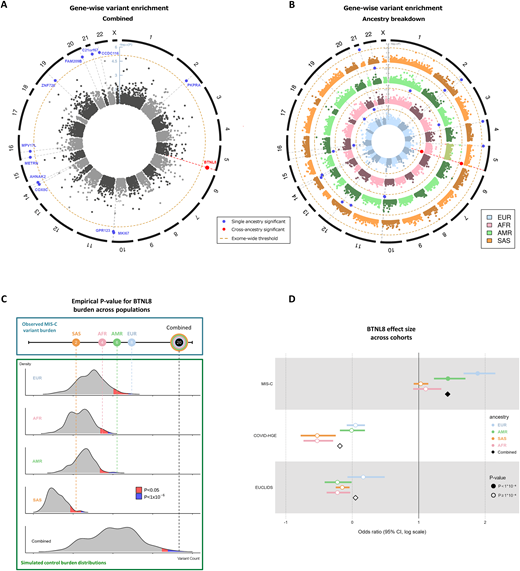
Multisystem inflammatory syndrome in children (MIS-C) is a rare condition following SARS-CoV-2 infection associated with intestinal manifestations. Genetic predisposition, including inborn errors of the OAS-RNAseL pathway, has been reported. We sequenced 154 MIS-C patients and utilized a novel statistical framework of gene burden analysis, “burdenMC,” which identified an enrichment for rare predicted-deleterious variants in BTNL8 (OR = 4.2, 95% CI: 3.5–5.3, P < 10-6). BTNL8 encodes an intestinal epithelial regulator of Vγ4+γδ T cells implicated in regulating gut homeostasis. Enrichment was exclusive to MIS-C, being absent in patients with COVID-19 or bacterial disease. Using an available functional test for BTNL8, rare variants from a larger cohort of MIS-C patients (n = 835) were tested which identified eight variants in 18 patients (2.2%) with impaired engagement of Vγ4+γδ T cells. Most of these variants were in the B30.2 domain of BTNL8 implicated in sensing epithelial cell status. These findings were associated with altered intestinal permeability, suggesting a possible link between disrupted gut homeostasis and MIS-C-associated enteropathy triggered by SARS-CoV-2.