2024-11-26 ロスアラモス国立研究所(LANL)
<関連情報>
- https://www.lanl.gov/media/news/1126-covid-mathematical-models
- https://www.pnas.org/doi/10.1073/pnas.2406303121
- https://www.nature.com/articles/s41591-022-01780-9
ヒトチャレンジ試験に基づくSARS-CoV-2感染の動態 The kinetics of SARS-CoV-2 infection based on a human challenge study
Sarafa A. Iyaniwura, Ruy M. Ribeiro, Carolin Zitzmann, +2, and Alan S. Perelson
Proceedings of the National Academy of Sciences Published:November 7, 2024
DOI:https://doi.org/10.1073/pnas.2406303121

Significance
Studying the early dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in humans is difficult. Here, we take advantage of a detailed dataset from a human challenge study to fit dynamic models that include innate and adaptive immune responses to longitudinal changes in both infectious and total virus. We uncovered a nonlinear relationship between total virus and infectious virus. We found that viral replication, after a short delay, is rapid, with a doubling time of ~2 h for viral RNA and ~3 h for infectious virus. We also found that innate immunity wanes as virus is brought under control, which together with adaptive immunity, initiated ~7 to 10 d postinfection, contributes to a multiphasic viral decline experienced by some participants.
Abstract
Studying the early events that occur after viral infection in humans is difficult unless one intentionally infects volunteers in a human challenge study. Here, we use data about severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in such a study in combination with mathematical modeling to gain insights into the relationship between the amount of virus in the upper respiratory tract and the immune response it generates. We propose a set of dynamic models of increasing complexity to dissect the roles of target cell limitation, innate immunity, and adaptive immunity in determining the observed viral kinetics. We introduce an approach for modeling the effect of humoral immunity that describes a decline in infectious virus after immune activation. We fit our models to viral load and infectious titer data from all the untreated infected participants in the study simultaneously. We found that a power-law with a power h < 1 describes the relationship between infectious virus and viral load. Viral replication at the early stage of infection is rapid, with a doubling time of ~2 h for viral RNA and ~3 h for infectious virus. We estimate that adaptive immunity is initiated ~7 to 10 d postinfection and appears to contribute to a multiphasic viral decline experienced by some participants; the viral rebound experienced by other participants is consistent with a decline in the interferon response. Altogether, we quantified the kinetics of SARS-CoV-2 infection, shedding light on the early dynamics of the virus and the potential role of innate and adaptive immunity in promoting viral decline during infection.
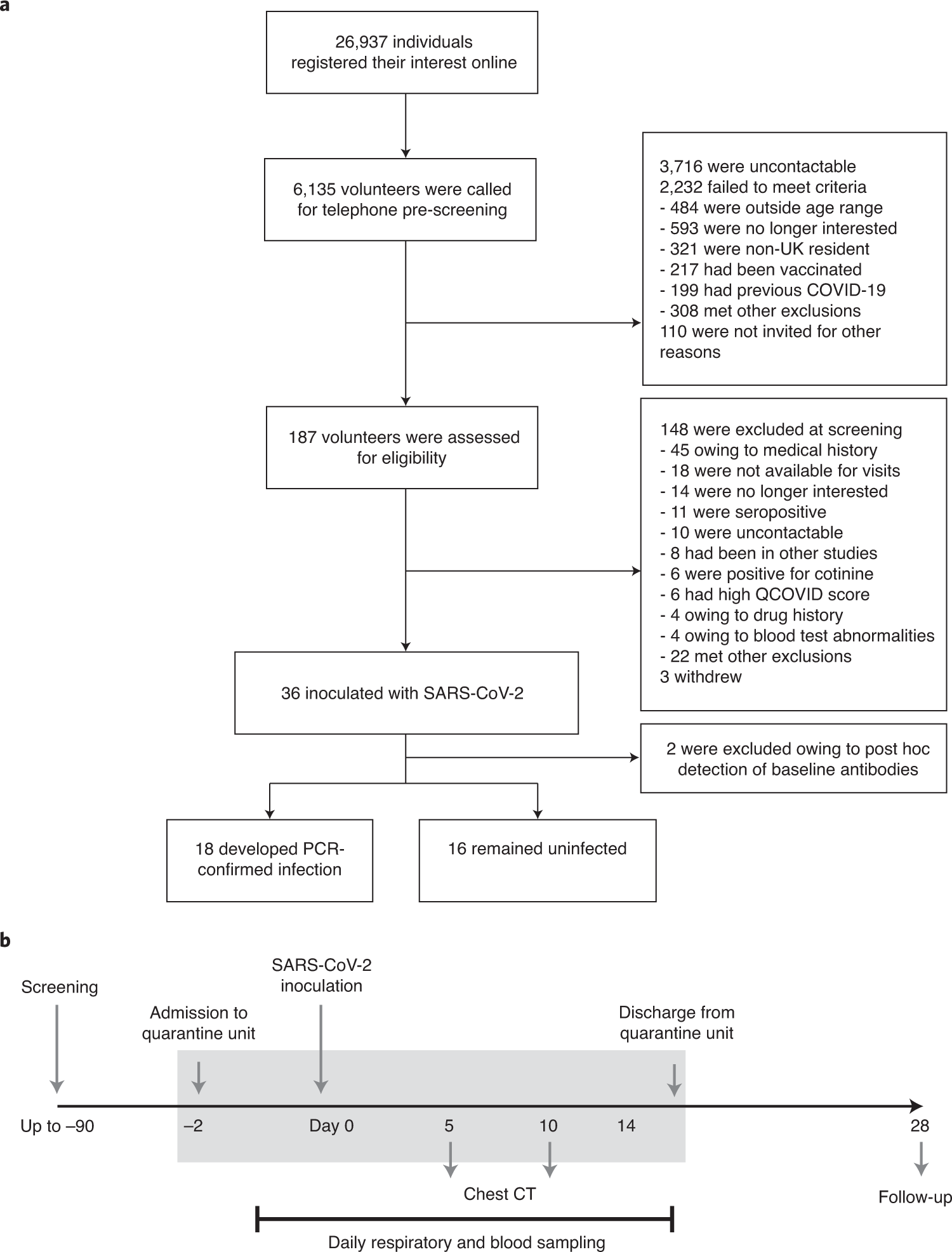
若年成人におけるSARS-CoV-2ヒトチャレンジ時の安全性、忍容性、ウイルス動態 Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults
Ben Killingley,Alex J. Mann,Mariya Kalinova,Alison Boyers,Niluka Goonawardane,Jie Zhou,Kate Lindsell,Samanjit S. Hare,Jonathan Brown,Rebecca Frise,Emma Smith,Claire Hopkins,Nicolas Noulin,Brandon Löndt,Tom Wilkinson,Stephen Harden,Helen McShane,Mark Baillet,Anthony Gilbert,Michael Jacobs,Christine Charman,Priya Mande,Jonathan S. Nguyen-Van-Tam,Malcolm G. Semple,… Christopher Chiu
Nature Medicine Published:31 March 2022
DOI:https://doi.org/10.1038/s41591-022-01780-9
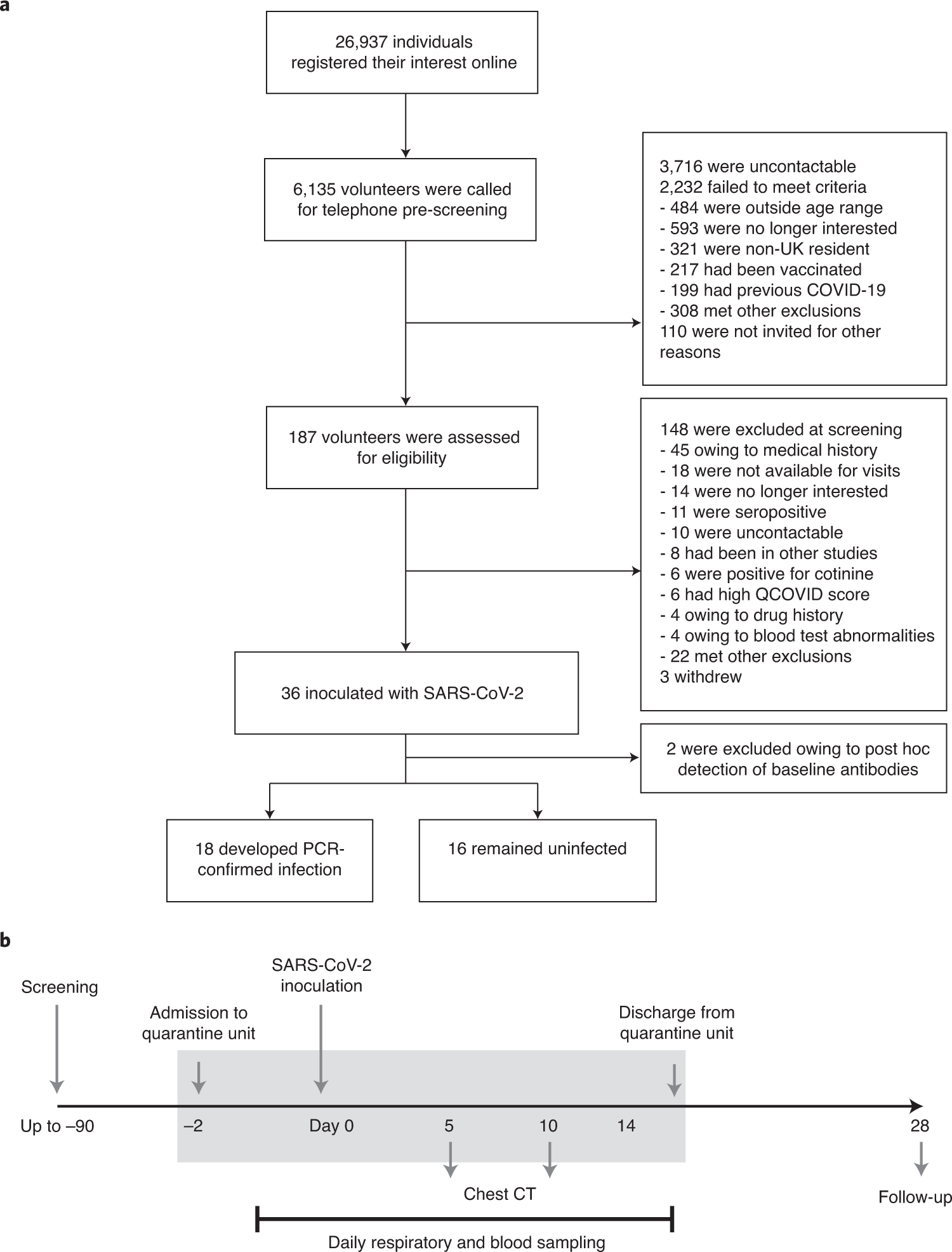
Abstract
Since its emergence in 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused hundreds of millions of cases and continues to circulate globally. To establish a novel SARS-CoV-2 human challenge model that enables controlled investigation of pathogenesis, correlates of protection and efficacy testing of forthcoming interventions, 36 volunteers aged 18–29 years without evidence of previous infection or vaccination were inoculated with 10 TCID50 of a wild-type virus (SARS-CoV-2/human/GBR/484861/2020) intranasally in an open-label, non-randomized study (ClinicalTrials.gov identifier NCT04865237; funder, UK Vaccine Taskforce). After inoculation, participants were housed in a high-containment quarantine unit, with 24-hour close medical monitoring and full access to higher-level clinical care. The study’s primary objective was to identify an inoculum dose that induced well-tolerated infection in more than 50% of participants, with secondary objectives to assess virus and symptom kinetics during infection. All pre-specified primary and secondary objectives were met. Two participants were excluded from the per-protocol analysis owing to seroconversion between screening and inoculation, identified post hoc. Eighteen (~53%) participants became infected, with viral load (VL) rising steeply and peaking at ~5 days after inoculation. Virus was first detected in the throat but rose to significantly higher levels in the nose, peaking at ~8.87 log10 copies per milliliter (median, 95% confidence interval (8.41, 9.53)). Viable virus was recoverable from the nose up to ~10 days after inoculation, on average. There were no serious adverse events. Mild-to-moderate symptoms were reported by 16 (89%) infected participants, beginning 2–4 days after inoculation, whereas two (11%) participants remained asymptomatic (no reportable symptoms). Anosmia or dysosmia developed more slowly in 15 (83%) participants. No quantitative correlation was noted between VL and symptoms, with high VLs present even in asymptomatic infection. All infected individuals developed serum spike-specific IgG and neutralizing antibodies. Results from lateral flow tests were strongly associated with viable virus, and modeling showed that twice-weekly rapid antigen tests could diagnose infection before 70–80% of viable virus had been generated. Thus, with detailed characterization and safety analysis of this first SARS-CoV-2 human challenge study in young adults, viral kinetics over the course of primary infection with SARS-CoV-2 were established, with implications for public health recommendations and strategies to affect SARS-CoV-2 transmission. Future studies will identify the immune factors associated with protection in those participants who did not develop infection or symptoms and define the effect of prior immunity and viral variation on clinical outcome.