2024-12-05 レンセラー工科大学 (RPI)
<関連情報>
- https://news.rpi.edu/2024/12/05/new-breakthrough-sheds-light-evolution-and-adaptability-photosynthetic-organisms-0#
- https://www.science.org/doi/10.1126/sciadv.adp4937
電子伝達鎖に珍しいプラストキノン誘導体を含む光化学系I変異体の低温電子顕微鏡構造 Cryo-EM structure of a photosystem I variant containing an unusual plastoquinone derivative in its electron transfer chain
Christopher J. Gisriel, Vasily Kurashov, David F. Iwig, Brandon P. Russell, […], and K. V. Lakshmi
Science Advances Published:29 Nov 2024
DOI:https://doi.org/10.1126/sciadv.adp4937

Abstract
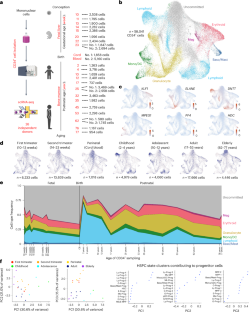
Photosystem I (PS I) is a light-driven oxidoreductase responsible for converting photons into chemical bond energy. Its application for renewable energy was revolutionized by the creation of the MenB deletion (ΔmenB) variant in the cyanobacterium Synechocystis sp. PCC 6803, in which phylloquinone is replaced by plastoquinone-9 with a low binding affinity. This permits its exchange with exogenous quinones covalently coupled to dihydrogen catalysts that bind with high affinity, thereby converting PS I into a stable solar fuel catalyst. Here, we reveal the 2.03-Å-resolution cryo-EM structure of a recent MenB variant of PS I. The quinones and their binding environment are analyzed in the context of previous biophysical data, thereby enabling a protocol to solve future PS I hybrids and constructs from this genetically tractable cyanobacterium.