2025-01-03 カリフォルニア大学サンディエゴ校(UCSD)
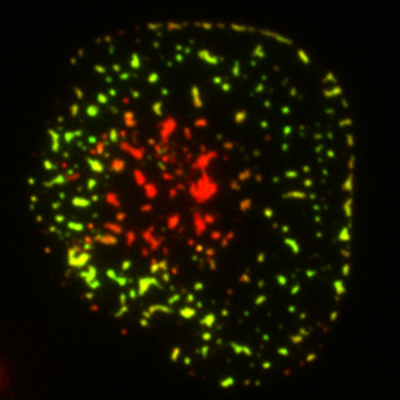
Microclusters: A fluorescent microscope image depicts the spatial pattern of an immunoreceptor (red) and its effector molecules (green) at the plasma membrane of a simulated T cell.
<関連情報>
- https://today.ucsd.edu/story/scientists-unveil-surprising-human-vs-mouse-differences-in-a-major-cancer-immunotherapy-target
- https://www.science.org/doi/10.1126/sciimmunol.ads6295
げっ歯類とヒトのPD-1の機能的相違は進化の分岐と関連する Functional differences between rodent and human PD-1 linked to evolutionary divergence
Takeya Masubuchi, Lin Chen, Nimi Marcel, George A. Wen, […], and Enfu Hui
Science Immunology Published:3 Jan 2025
DOI:https://doi.org/10.1126/sciimmunol.ads6295
Editor’s summary
Preclinical studies in mice have been critical to understanding how the inhibitory receptor PD-1 functions. However, human and murine PD-1 share less than 60% amino acid identity. Masubuchi et al. found that human PD-1 was more inhibitory compared with murine PD-1 because of stronger binding interactions with its ligands PD-L1 and PD-L2 and more effective recruitment of the Shp2 phosphatase. A motif highly conserved across PD-1 orthologs in vertebrates but absent from rodents promoted Shp2 interactions. Evolutionary analyses suggest that ancient rodents lost this motif in PD-1 after a major extinction event. Humanization of this motif or the intracellular domain of PD-1 reduced tumor-specific T cell responses in mice. These findings define key distinctions of rodent and human PD-1 orthologs. —Christiana N. Fogg
Abstract
Mechanistic understanding of the inhibitory immunoreceptor PD-1 is largely based on mouse models, but human and mouse PD-1 share only 59.6% amino acid identity. Here, we found that human PD-1 is more inhibitory than mouse PD-1, owing to stronger interactions with the ligands PD-L1 and PD-L2 and more efficient recruitment of the effector phosphatase Shp2. In a mouse melanoma model with adoptively transferred T cells, humanization of a PD-1 intracellular domain disrupted the antitumor activity of CD8+ T cells and increased the magnitude of anti–PD-1 response. We identified a motif highly conserved across vertebrate PD-1 orthologs, absent in rodents, as a key determinant for differential Shp2 recruitment. Evolutionary analysis suggested that PD-1 underwent a rodent lineage–specific functional attenuation during evolution. Together, our study uncovers species-specific features of the PD-1 pathway, with implications for PD-1 evolution and differential anti–PD-(L)1 responses in mouse models and human patients.