2025-02-24 カリフォルニア工科大学(Caltech)
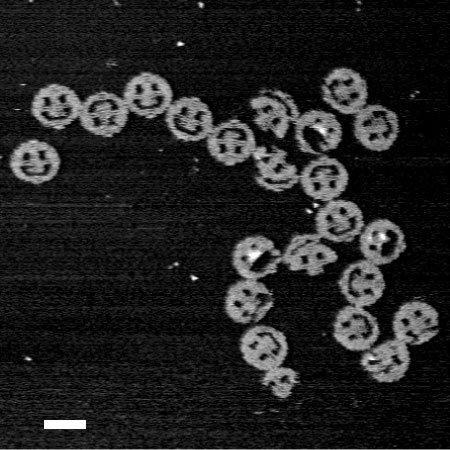
DNA origami smiley faces, each 1/1000 the width of a human hair, demonstrate that virtually any shape can be folded from DNA. (atomic force microscopy image; scale bar: 100 nanometers)Credit: Paul W.K. Rothemund/Caltech
<関連情報>
- https://www.caltech.edu/about/news/dna-origami-suggests-route-to-reusable-multifunctional-biosensors
- https://www.pnas.org/doi/10.1073/pnas.2311279121
- https://www.nature.com/articles/nature04586
モジュラーDNA折り紙を用いたDNAとタンパク質の電気化学的検出 Modular DNA origami–based electrochemical detection of DNA and proteins
Byoung-jin Jeon, Matteo M. Guareschi, Jaimie Marie Stewart, +6, and Paul W. K. Rothemund
Proceedings of the National Academy of Sciences Published:December 30, 2024
DOI:https://doi.org/10.1073/pnas.2311279121
Significance
Aptamer-based, conformation-switching electrochemical biosensors enable real-time analyte sensing in the living body. However, developing sensors for new analytes is limited by the difficulty of optimizing electrochemical signal strength and gain, without compromising binding properties of the aptamer. Optimization of new sensors often requires synthesis of many expensive, multiply modified oligonucleotides. In contrast, we describe a reagentless DNA origami-based platform whose binding and signaling properties are essentially independent. This independence enables signal optimization to be based on analyte size; sensors are customized using a library of inexpensive and unmodified DNA linkers. This library is portable between different sensor designs and analytes. Our work thus provides a “toolkit” for constructing electrochemical biosensors, and a general approach for modular biosensors beyond electrochemical detection.
Abstract
The diversity and heterogeneity of biomarkers has made the development of general methods for single-step quantification of analytes difficult. For individual biomarkers, electrochemical methods that detect a conformational change in an affinity binder upon analyte binding have shown promise. However, because the conformational change must operate within a nanometer-scale working distance, an entirely new sensor, with a unique conformational change, must be developed for each analyte. Here, we demonstrate a modular electrochemical biosensor, built from DNA origami, which is easily adapted to diverse molecules by merely replacing its analyte binding domains. Instead of relying on a unique nanometer-scale movement of a single redox reporter, all sensor variants rely on the same 100-nm scale conformational change, which brings dozens of reporters close enough to a gold electrode surface that a signal can be measured via square-wave voltammetry, a standard electrochemical technique. To validate our sensor’s mechanism, we used single-stranded DNA as an analyte, and optimized the number of redox reporters and various linker lengths. Adaptation of the sensor to streptavidin and Platelet-Derived Growth Factor-BB (PDGF-BB) analytes was achieved by simply adding biotin or anti-PDGF aptamers to appropriate DNA linkers. Geometrically optimized streptavidin sensors exhibited signal gain and limit of detection markedly better than comparable reagentless electrochemical sensors. After use, the same sensors could be regenerated under mild conditions: Performance was largely maintained over four cycles of DNA strand displacement and rehybridization. By leveraging the modularity of DNA nanostructures, our work provides a straightforward route to the single-step quantification of arbitrary nucleic acids and proteins.
DNAを折り曲げてナノスケールの形とパターンを作る Folding DNA to create nanoscale shapes and patterns
Paul W. K. Rothemund
Nature Published:16 March 2006
DOI:https://doi.org/10.1038/nature04586
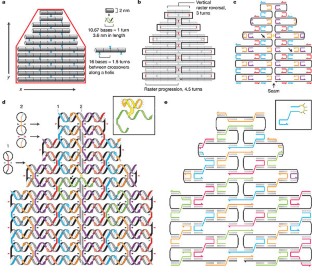
Abstract
‘Bottom-up fabrication’, which exploits the intrinsic properties of atoms and molecules to direct their self-organization, is widely used to make relatively simple nanostructures. A key goal for this approach is to create nanostructures of high complexity, matching that routinely achieved by ‘top-down’ methods. The self-assembly of DNA molecules provides an attractive route towards this goal. Here I describe a simple method for folding long, single-stranded DNA molecules into arbitrary two-dimensional shapes. The design for a desired shape is made by raster-filling the shape with a 7-kilobase single-stranded scaffold and by choosing over 200 short oligonucleotide ‘staple strands’ to hold the scaffold in place. Once synthesized and mixed, the staple and scaffold strands self-assemble in a single step. The resulting DNA structures are roughly 100 nm in diameter and approximate desired shapes such as squares, disks and five-pointed stars with a spatial resolution of 6 nm. Because each oligonucleotide can serve as a 6-nm pixel, the structures can be programmed to bear complex patterns such as words and images on their surfaces. Finally, individual DNA structures can be programmed to form larger assemblies, including extended periodic lattices and a hexamer of triangles (which constitutes a 30-megadalton molecular complex).


