2025-02-21 清華大学
<関連情報>
- https://www.tsinghua.edu.cn/en/info/1245/14098.htm
- https://www.nature.com/articles/s41586-024-08395-9
GZMKを発現するCD8+ T細胞が気道炎症性疾患の再発を促進する GZMK-expressing CD8+ T cells promote recurrent airway inflammatory diseases
Feng Lan,Jizhou Li,Wenxuan Miao,Fei Sun,Su Duan,Yabing Song,Jiacheng Yao,Xiangdong Wang,Chengshuo Wang,Xin Liu,Jianbin Wang,Luo Zhang &Hai Qi
Nature Published:15 January 2025
DOI:https://doi.org/10.1038/s41586-024-08395-9
Abstract
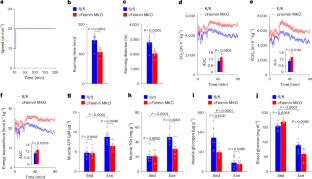
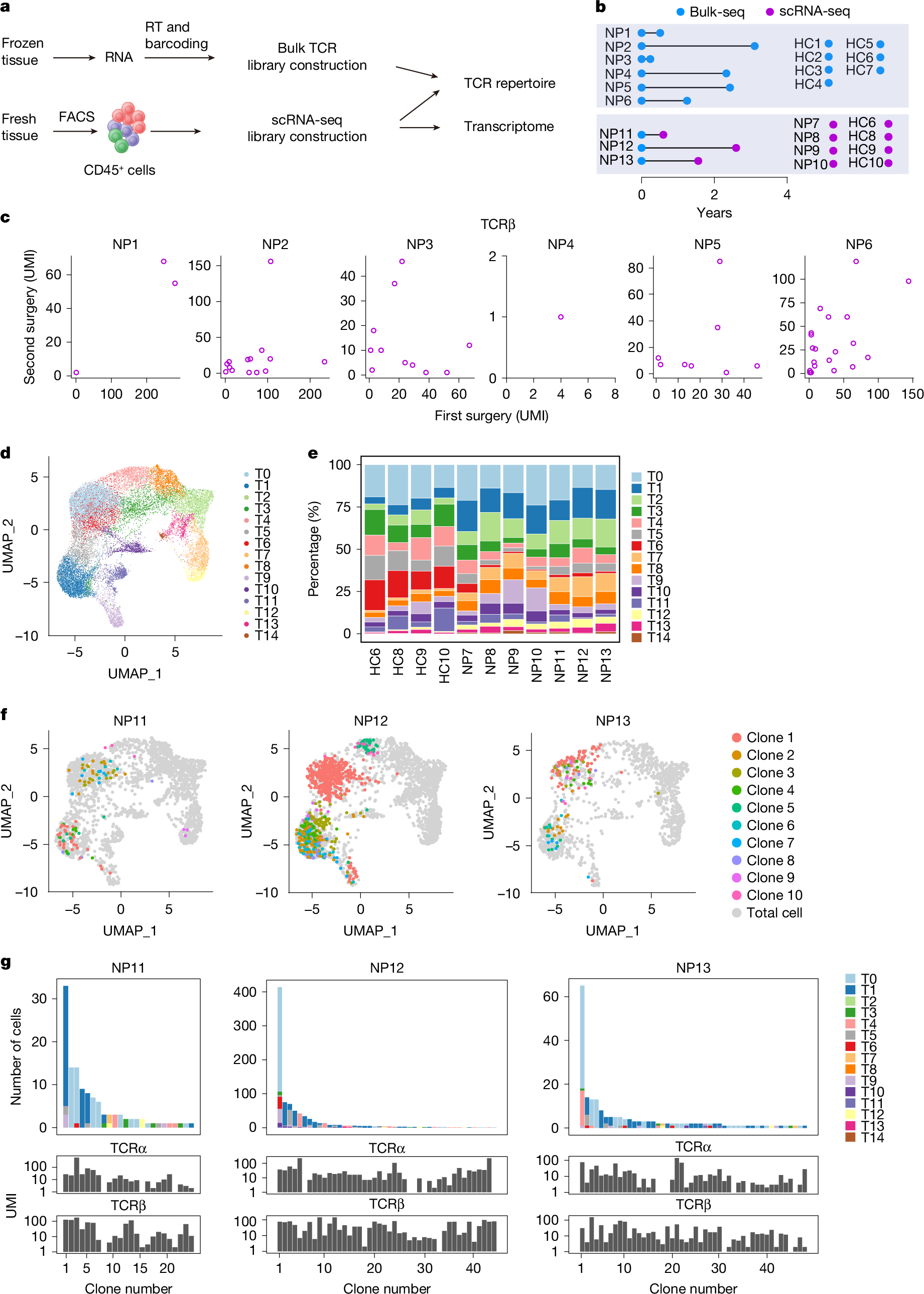
Inflammatory diseases are often chronic and recurrent, and current treatments do not typically remove underlying disease drivers. T cells participate in a wide range of inflammatory diseases such as psoriasis, Crohn’s disease, oesophagitis and multiple sclerosis, and clonally expanded antigen-specific T cells may contribute to disease chronicity and recurrence, in part by forming persistent pathogenic memory. Chronic rhinosinusitis and asthma are inflammatory airway diseases that often present as comorbidities. Chronic rhinosinusitis affects more than 10% of the general population. Among these patients, 20–25% would develop nasal polyps, which often require repeated surgical resections owing to a high incidence of recurrence. Whereas abundant T cells infiltrate the nasal polyps tissue, T cell subsets that drive the disease pathology and promote recurrence are not fully understood. By comparing T cell repertoires in nasal polyp tissues obtained from consecutive surgeries, here we report that persistent CD8+ T cell clones carrying effector memory-like features colonize the mucosal tissue during disease recurrence, and these cells characteristically express the tryptase Granzyme K (GZMK). We find that GZMK cleaves many complement components, including C2, C3, C4 and C5, that collectively contribute to the activation of the complement cascade. GZMK-expressing CD8+ T cells participate in organized tertiary lymphoid structures, and tissue GZMK levels predict the disease severity and comorbidities better than well-established biomarkers such as eosinophilia and tissue interleukin-5. Using a mouse asthma model, we further show that GZMK-expressing CD8+ T cells exacerbate the disease in a manner dependent on the proteolytic activity of GZMK and complements. Genetic ablation or pharmacological inhibition of GZMK after the disease onset markedly alleviates tissue pathology and restores lung function. Our work identifies a pathogenic CD8+ memory T cell subset that promotes tissue inflammation and recurrent airway diseases by the effector molecule GZMK and suggests GZMK as a potential therapeutic target.