2025-03-11 京都大学
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-03-11
- https://www.kyoto-u.ac.jp/sites/default/files/2025-03/web_2503_Noda-f9a1ee71da9ef367f19bcd35aa10a780.pdf
- https://www.nature.com/articles/s41467-025-57236-4
エボラウイルスのヌクレオキャプシド形成とVP24が制御する機能の構造的基盤 Structural basis for Ebola virus nucleocapsid assembly and function regulated by VP24
Yoko Fujita-Fujiharu,Shangfan Hu,Ai Hirabayashi,Yuki Takamatsu,Yen Ni Ng,Kazuya Houri,Yukiko Muramoto,Masahiro Nakano,Yukihiko Sugita & Takeshi Noda
Nature Communications Published:10 March 2025
DOI:https://doi.org/10.1038/s41467-025-57236-4
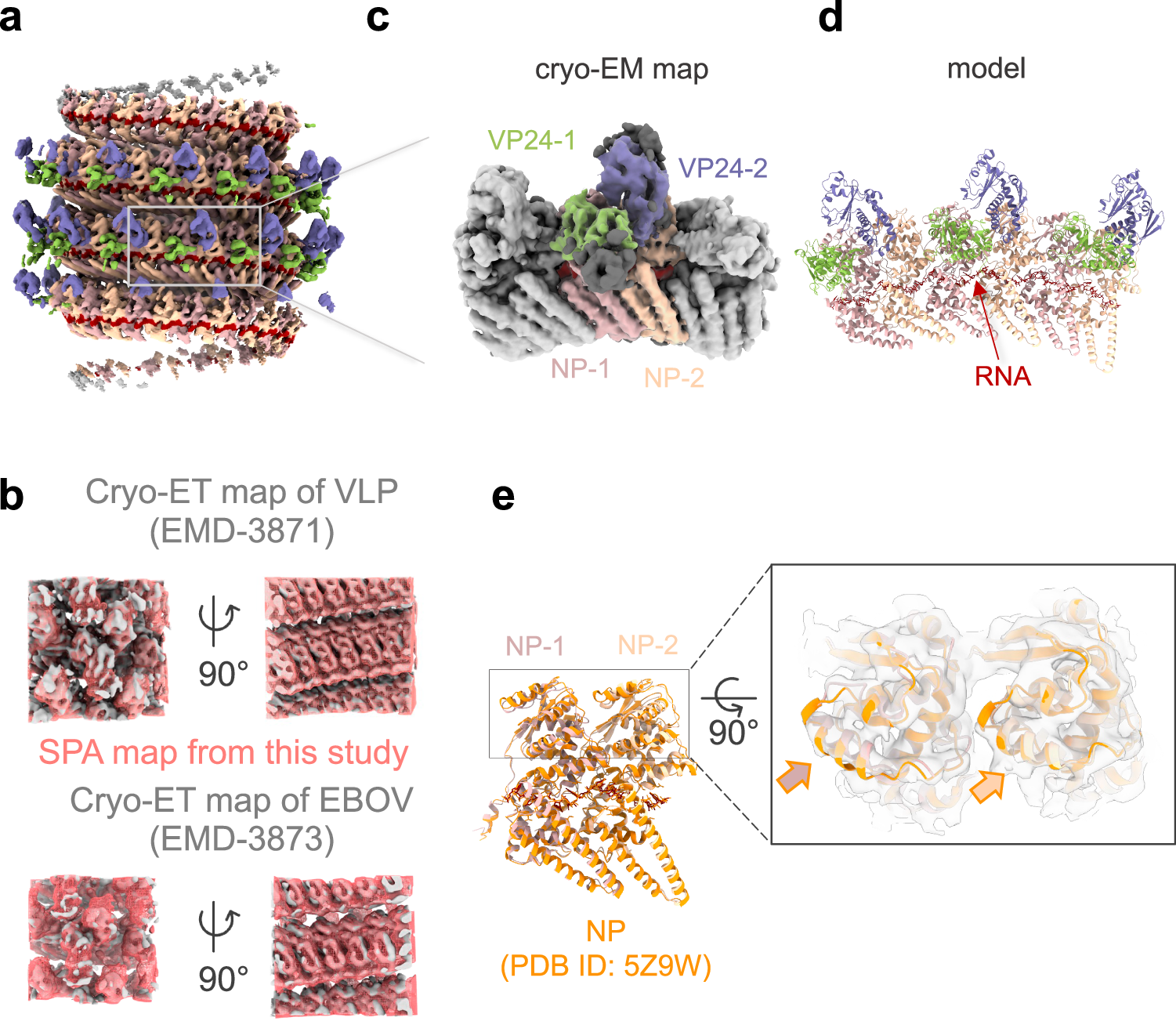
Abstract
The Ebola virus, a member of the Filoviridae family, causes severe hemorrhagic fever in humans. Filamentous virions contain a helical nucleocapsid responsible for genome transcription, replication, and packaging into progeny virions. The nucleocapsid consists of a helical nucleoprotein (NP)–viral genomic RNA complex forming the core structure, to which VP24 and VP35 bind externally. Two NPs, each paired with a VP24 molecule, constitute a repeating unit. However, the detailed nucleocapsid structure remains unclear. Here, we determine the nucleocapsid-like structure within virus-like particles at 4.6 Å resolution using single-particle cryo-electron microscopy. Mutational analysis identifies specific interactions between the two NPs and two VP24s and demonstrates that each of the two VP24s in different orientations distinctively regulates nucleocapsid assembly, viral RNA synthesis, intracellular transport of the nucleocapsid, and infectious virion production. Our findings highlight the sophisticated mechanisms underlying the assembly and functional regulation of the nucleocapsid and provide insights into antiviral development.